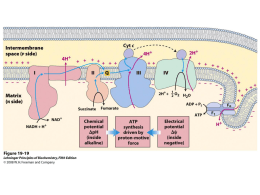
Indian Journal of Biochemistry & Biophysics Vol. 51, October 2014, pp. 365-371 Energy restriction and impact on indirect calorimetry and oxidative stress in cardiac tissue in rat Elisa Ito Kawahara, Nadine Helena Pelegrino Bastos Maués, Klinsmann Carolo dos Santos, Pedro Octávio Barbanera, Camila Pereira Braga and Ana Angélica Henrique Fernandes1,* Department of Chemistry and Biochemistry, Institute of Bioscience, São Paulo State University, UNESP, Botucatu, CEP 18618-970, São Paulo, Brazil Received 11 October 2013; revised 19 August 2014 Caloric restriction, defined as a reduction in calorie intake below ad libitum, without malnutrition can have beneficial effects. In this study, we evaluated the impact of caloric restriction of 30 and 60% on calorimetric parameters and oxidative stress in cardiac tissue in rats. Rats were randomly divided into 3 groups (n = 8): G1 = control; G2 = rats exposed to dietary restriction of 30%; and G3 = rats exposed to dietary restriction of 60%. Energy restriction decreased final body weight, oxidation of carbohydrates and lipid, oxygen consumption (VO2), carbon dioxide production (VCO2), resting metabolic rate (RMR), but elevated respiratory quotient (RQ). G3 animals also displayed an imbalance in the oxidant/antioxidant system, as revealed by the decrease in the lipid hydroperoxide (LH) level and GSH-Px activity in heart tissue. In conclusion, dietary restriction decreased oxidative metabolism, as seen by the colorimetric profiles and controlled oxidative stress in cardiac tissue. Keywords: Calorimetry, Nutritional parameters, Oxidative stress, Energy restriction, Myocardium. Dietary restriction can positively influence the longevity of animals and also decrease the progression and impact of degenerative chronic diseases1,2. More specifically, caloric restriction, defined as a reduction in calorie intake below ad libitum, without malnutrition and altered normal levels of macronutrients can provide a nutritional intervention that extends the life span of a variety of species, including mammals3. McCay et al4 first published the effects of caloric restriction in mice. They noted that caloric restriction after puberty in the mice led to prolonged life and mitigation of age-related diseases. In rodents, caloric reduction of 60% immediately after puberty (six months) increases longevity by 30 to 60%, while a reduction of 44% of caloric intake in adulthood (12 months) extends the maximum life expectancy by only 10 to 20%5. —————— *Corresponding author E-mail: [email protected] or [email protected] Phone: + 55 (14) 3880-0644 Abbreviations: BW, body weight; CI, carbohydrate intake; VCO2, carbon dioxide production; EI, energy intake; FE, feed efficiency; GSH-Px, glutatione peroxidase; LH, lipid hydroperoxide; LI, lipid intake; VO2, oxygen consumption; PI, protein intake; ROS, reactive oxygen species, RQ, respiratory quotient; RMR, resting metabolic rate; SOD, superoxide dismutase. It has been proposed that food restriction attenuates the generation of reactive oxygen species (ROS), consequently decreasing lipoperoxidation, avoiding structural changes of the cellular membrane and preventing the accumulation of oxidized macromolecules, especially proteins and DNA; these events prevent cell death and lead to an increase in the life span6. Caloric restriction is also reported to elicit an adaptive reduction in energy expenditure through mechanisms, such as reduced motor activity, body weight, basal metabolism, thermogenesis and fat-increased energy efficiency7,8. Carbohydrate restriction also results in metabolic adaptations, which subsequently lead to lipid metabolism. This is caused by increased secretion of lipolytic hormones (epinephrine, cortisol and growth hormone) in response to decreased glucose and insulin levels9. Lipolysis is stimulated in adipose tissue and, therefore, increases the availability of circulating free fatty acids for tissues. In addition, substrates are available for gluconeogenesis, such as glycerol, which is derived from tissue adipose, lactate and alanine formed in the muscle10. The heart has an essentially aerobic metabolism with elevated oxidation of carbohydrate and fatty acid 366 INDIAN J. BIOCHEM. BIOPHYS., VOL. 51, OCTOBER 2014 β-oxidation, resulting in reducing equivalents (NADH and FADH2), which reoxide in the electron transport chain (ETC) through oxidative phosphorylation11,12. Evidence shows that during excessive electron transport the formation of ROS, such as superoxide anion occurs11,12. There is association between increased mitochondrial b-oxidation and ROS production13,14. It provokes mitochondrial uncoupling, which is implicated with decrease in the production of ATP and consequent cellular injury, leading to cardiac dysfunction15,16. Thus, restricting the oxidizable substrate uptake could prevent excessive oxidation of lipid and glucose and control the amount of electrons from reducing equivalents NADH and FADH in the ETC and consequently decrease the ROS generation. This strategy would avoid cardiac mitochondrial dysfunction, ensuring adequate supply of ATP to the heart. Although there are several studies available on caloric restriction and the aging process, but reports on the impact of caloric restriction on oxidative stress and calorimetric parameters in cardiac tissue are lacking. Therefore, in this study, we have evaluated the effect of caloric restriction (30 and 60%) on calorimetric parameters and oxidative stress in the cardiac tissue in rats. Materials and Methods Experimental design The experimental protocol (No. 479/2013) was approved by the Ethics Committee on use of animal in the experimentation of the Bioscience Institute (UNESP/Botucatu, São Paulo) and all animals were cared for in accordance with the principles and guidelines of the Canadian Council on Animal Care. Male Wistar rats (30-day old) weighing ± 250 g were maintained under controlled environmental conditions (22 ± 2°C, 40 to 50% humidity and 12 h light/dark cycle). Animals were randomly divided into three groups (n = 8). The control group (G1) received a standard diet (38.0% fat, 44.5% carbohydrate, 20.0% protein and 3.0 kcal/g of metabolisable energy; Purina, Campinas, São Paulo, Brazil) ad libitum. The experimental groups were exposed to dietary restriction, which was 30% (Group 2 or G2) and 60% (Group 3 or G3) of the amount of food consumed by the rats in the control group. Food consumption was determined daily at the same time (9:00-10:00 am) and calculated by subtracting the food offered and that remaining after consumption in 24 h. At the end of experimental period (30 days), the animals were fasted overnight (14), anaesthetised with a mixture (v/v) of xylazine and ketamine and sacrificed by decapitation. Total blood was collected and serum was separated by centrifugation at 6,000 rpm for 15 min. The heart was retrieved, weighed and stored at -80°C. Determination of total protein The protein content was determined with release of the end product whose intensity of color was proportional to concentration of the total protein present in the sample and measured at 540 nm17. Measurement of oxidative stress Cardiac tissue (200 mg of left ventricle) was homogenised (0.1 M phosphate buffer; pH 7.4) and centrifuged at 10.000 rpm for 15 min. The supernatant was used to analyze total protein levels, as well as the activities of lipid hydroperoxide (LH), glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and catalase. The determination of LH was based on the principle of hydroperoxide-mediated oxidation of Fe2+ in the presence of xylenol orange, FeSO4, H2SO4 and butylated hydroxytoluene in 90% (v/v) methanol. The end product was read at 560 nm18. The activity of GSH-Px was assayed at pH 7.0 (phosphate buffer) in medium containing EDTA, NADPH, gluthathione reductase, sodium azide and the reduced form of glutathione. The absorbance of the reaction was measured at 340 nm19. The sample containing SOD was mixed with phosphate buffer (pH 7.4), EDTA, nitroblue tetrazolium (NBT), NADH and phenazine methosulphate. SOD activity was proportional to the amount of the reduced form of NBT. The absorbance was measured at 560 nm20. Catalase activity was determined with phosphate buffer (pH 7.0) and hydrogen peroxide. Absorbance was recorded at 340 nm21. The values for enzyme activities were obtained using a microplate reader (µQuant-MQX 200 with Kcjunior software, Bio-Tec Instruments, Winooski, VT, USA). All reagents were purchased from Sigma, St. Louis, MO, USA. Nutritional parameters Based on food intake and the amount of calories (Table 1), the following parameters were calculated: energy intake (EI; kcal/day) = average daily consumption of feed ration × metabolisable energy of the ration in kcal/g; feed efficiency (FE;%) = weight gain (g)/energy intake (kcal) × 100; protein intake (PI; g/day) = average daily consumption of feed ration KAWAHARA et al.: ENERGY RESTRICTION IMPACT IN CARDIAC TISSUE × percentage of feed protein; lipid intake (LI; g/day) = average daily consumption of feed ration × percentage of feed lipids; and carbohydrate intake (CI; g/day) = average daily consumption of feed ration × percentage of carbohydrates in the diet22,23. Body weight was measured once weekly. Indirect calorimetry At the end of experimental period, respiratory quotient (RQ) and energy expenditure, i.e., resting metabolic rate (RMR), average oxygen consumption (V02) and carbon dioxide production (VC02) within a 40 min period were evaluated in rats fasted overnight (12 to 14 hr). The measurements were taken by a computer-monitored indirect calorimeter (CWE, Inc, St. Paul, USA) coupled to metabolic chambers (air flow = 1.01/min), which accommodated the rats fasting overnight (12 to 14 h). The calorimetric parameters were measured using a respiratory-based software program (software MMX, CWE, Inc., USA). The calorimetric parameters were calculated through the relative oxidative proportions of the oxidation and the amount of oxygen consumed per g of oxidised substrate, carbohydrate and fat oxidation using the equations: VO2 × (RQ - 0.707)/0.293 × 0.746 (for carbohydrate oxidation) and VO2 × (1 - RQ)/0.293 × 0.746 (for fat oxidation), where VO2 is measured as l/min, 1.00 is the RQ for total carbohydrate oxidation, 0.707 is the RQ for total fat Table 1—Body weight final, food consumption, energy intake, food efficiency, carbohydrate intake, protein and lipid intake in control (G1), 30% (G2) and 60% (G3) energy restriction groups [Values expressed as mean ± SD] Parameters G1 G2 G3 Body weight 421.68 ± 24.57c 336.36 ± 28.47b 246.69 ± 22.22a (g) Food 25.04 ± 1.19c 17.70 ± 0.58b 10.09 ± 0.34a consumption (g) Energy intake 95.44 ± 4.54c 67.45 ± 2.22b 38.46 ± 1.32a (kcal/day) Food efficiency 0.97 ± 0.05c 0.88 ± 0.03b 0.57 ± 0.02a (g/kcal) Carbohydrate 0.29 ± 0.01c 0.20 ± 0.01b 0.12 ± 0.00a intake (g/day) 0.94 ± 0.04c 0.67 ± 0.02b 0.38 ± 0.01a Protein intake (g/day) Lipid intake 21.50 ± 1.02c 15.19 ± 0.50b 8.66 ± 0.30a (g/day) a,b,c Means followed by different letters indicate significant differences between groups (p <0.05). 367 oxidation, 0.293 is the difference between 1.000 and 0.746 and is the number of litres of oxygen consumed per g of oxidised glucose24. Statistical analysis We used a completely randomised design with 3 treatments and 8 replications of the ANOVA scheme. The mean values of treatments were compared using Tukey’s test at 5% probability, according to Zar25. Results Significant decrease in body weight and nutritional parameters was observed in groups G2 and G3 rats, compared to the control group (Table 1). Animals undergoing energy restriction of 60% had the lowest (p<0.05) body weight at the end of the experimental period. Energy intake and food efficiency (p<0.05) decreased in the energy-restricted groups (G2 = 30% and G3 = 60%), compared to the control group (G1). The animals subjected to energy restriction of 60% (G3) showed lower values (p<0.05) for energy consumption, as well as total levels of carbohydrates, protein and lipids, when compared to the control. Table 2 shows data on indirect calorimetry. Animals subjected to energy restriction (G1 and G2) Table 2—Calorimetric parameters in control (G1), 30% (G2) and 60% (G3) energy restriction groups [Values expressed as mean ± SD] Parameters VO2/body surface (mL/h.g0.7) VO2 (mL/min) VCO2 (mL/min) RQ Oxidation of carbohydrate (mg/kg min) Oxidation of lipid (mg/kg min) RMR (kcal/h) Cardiac protein (mg/100 mg tissue) Serum total protein (g/dL) Heart weight/Corporal (g/kg) a,b,c G1 G2 6.85 ± 1.95b 4.51 ± 0.54a G3 3.75 ± 0.9a 3.80 ± 1.08b 2.50 ± 0.30a 2.08 ± 0.52a 2.25 ± 0.26b 2.08 ± 0.26b 1.51 ± 0.41a 0.62 ± 0.13a 0.83 ± 0.07c 0.72 ± 0.06b 1.10 ± 1.60c 0.79 ± 0.43b 0.09 ± 0.35a 3.95 ± 2.38b 1.08 ± 0.50a 1.46 ± 0.48a 1.04 ± 0.26b 0.72 ± 0.08a 0.58 ± 0.14a 23.17 ± 0.8c 17.07 ± 0.5b 14.18 ± 0.6a 9.38 ± 1.23a 9.12 ± 0.50a 9.11 ± 1.09a 2.61 ± 0.18a 2.59 ± 0.2a 2.18 ± 0.16a Means followed by different letters indicate significant differences between groups (p <0.05). VO2, oxygen consumption; VCO2, carbon dioxide production; RQ, respiratory quotient; RMR, resting metabolic rate. INDIAN J. BIOCHEM. BIOPHYS., VOL. 51, OCTOBER 2014 368 Table 3—Protein and oxidative stress markers in control (G1), 30% (G2) and 60% (G3) energy restriction groups [Values expressed as mean ± SD] Parameters G1 G2 G3 LH (nmol/g tissue) 145.97 ± 3.5c 93.41 ± 2.0b 70.18 ± 2.7a Catalase (µmol/g tissue) 120.09 ± 4.7b 84.27 ± 2.4a 79.95 ± 2.6a SOD (nmol/mg pt) 28.53 ± 1.07c 15.03 ± 1.8a 10.34 ± 1.0a GSH-Px (nmol/mg tissue) 59.03 ± 3.17b 57.48 ± 2.9b 21.61 ± 1.08a a,b,c: Means followed by different letters indicate significant differences between groups (p <0.05). LH, lipid hydroperoxide; SOD, superoxide dismutase; GSH-Px, glutathione peroxidase. showed decreased (p<0.05) VO2/surface body, oxygen consumption (VO2), CO2 production (VCO2), resting metabolic rate (RMR) and carbohydrate and lipid oxidation, compared to those who had free access to food. Respiratory quotient (RQ) increased in rats subjected to energy restriction, indicating fat oxidation. Data related to oxidative stress markers in cardiac tissue in control and energy restrictive groups are shown in Table 3. Animals of the groups G2 and G3 rats showed a decrease (p <0.05) in the activities of catalase and SOD, as well as a reduction of the total protein level in heart tissue, when compared to G1 animals. GSH-Px activity decreased only in animals undergoing 60% energy restriction. No differences (p > 0.05) were observed in the heart-to-body weight ratio among the groups. Discussion The changes in eating habits are an important factor in altering the oxidant/antioxidant balance in the body22,26. In this study, we evaluated the effect of energy restriction of 30 and 60% on indirect calorimetry and oxidative stress in cardiac tissue in rats. Caloric restriction in G2 and G3 rats caused the lowest weight at the end of the experimental period and lower values of calorimetric parameters, compared to control (G1) animals. The VO2 is the amount of oxygen consumed and VCO2 the amount of CO2 produced per g of metabolic substrate oxidised in the body. Using these parameters, it is possible to compare specific oxygen consumption and CO2 production in different species and animal tissues, since they reflect the gas exchange associated with the oxidation of nutrients in relation to body weight27. As lipid oxidation requires greater oxygen consumption, there was an increase in VO2 adjusted for body surface area in the energy restricted animals. As the chemical composition of carbohydrates (C6H12O6) differs from that of lipids [CH3-(CH2) n-COOH]), which contain more hydrogen and carbon atoms than oxygen ones per molecule, the use of oxygen in the oxidation of lipids is higher. Consequently, lipid oxidation yields more ATP molecules than carbohydrate oxidation28. Thus, lipid oxidation requires proportionally higher oxygen consumption and releases less CO2. The animals subjected to energy restriction had low oxygen uptake (VO2), indicating low lipid oxidation. Moreover, lower CO2 production (VCO2) suggested lower oxidation of carbohydrates in rats exposed to energy restriction of 60%, probably due to lower lipid and carbohydrate uptake. Since carbohydrates are polyhydroxyl aldehydes or ketones, structurally there is an oxygen atom for carbon for every three-carbon atom. Therefore, consumption of just one external oxygen atom is necessary to form a CO2 molecule during carbohydrate oxidation. Blood glucose level correlates positively with carbohydrate oxidation and negatively with fatty acid oxidation. In the present study, carbohydrate oxidation decreased with decreasing carbohydrate uptake. This was consistent with the results obtained in as earlier study29, since selection of metabolic fuel by the cell is controlled by carbohydrate uptake. Although fatty acids become the main metabolic fuel during energy deprivation due to increased lipolysis, in the present study, neither 30 nor 60% restriction was sufficient in enhancing lipid oxidation. As the cardiac and skeletal muscle tissues use a large part of the lipids consumed from a diet, low lipid oxidation may be a consequence of reduced lipid uptake. The amount of oxygen required during complete oxidation of macronutrients, especially fatty acids and carbohydrates is compatible with the produced quantity of CO2. Thus, RQ establishes a relationship between the CO2 produced and O2 consumed, thereby quantifying the mix of catabolized substrate to obtain energy. Hence, RQ was employed to determine the type of substrate being oxidized by the organism. A reduction in RQ indicates increased use of lipids as the energy substrate30,31. KAWAHARA et al.: ENERGY RESTRICTION IMPACT IN CARDIAC TISSUE The elevated RQ in animals subjected to energy restriction was due to a higher carbohydrate than lipid oxidation. This increase was higher in rats subjected to a 30% energy restriction due to the lower drop in carbohydrate oxidation, compared to those with 60% energy restriction, whose lipid oxidation remained unaltered. Animals that underwent energy restriction of 60% (G3) showed significantly lower RQ values than those exposed to a restriction of 30% (G2). However, there were no differences in oxygen consumption adjusted for body surface area, resting metabolic rate and lipid oxidation between these two groups. The significant reduction of the RQ in G3 rats compared to G2 animals indicated an increase in lipid oxidation at the expense of carbohydrate oxidation. Thus, the RQ was the lowest in G1 animals, while the values of VO2, VO2/surface body and VCO2 were the highest in this group, compared to G2 and G3 animals. This indicated that G1 animals used the most carbohydrates as the energy substrate, correlating with their final body weight, which was the highest among the three groups32. Comparing G2 and G3 rats, the latter group displayed the lowest final body weight, which also correlated with their lowest utilization of carbohydrate. Since the execution of vital functions in the waking period depends on a certain energy demand, the basal metabolic rate (RMR) accounts for the sum of cellular metabolic processes needed to preserve physiological functions. Thus, knowledge of the RMR allows the establishment of the energy level needed to develop body mass control strategies by means of energy restriction33. A reduced RMR may indicate a higher energy balance, i.e., low use of the ingested energy for basal metabolism and consequently higher availability for storage in adipose tissues, leading to body weight gain34. However, in our study, a decreased RMR in animals subjected to energy restriction did not increase body weight. This might be due to oxygen uptake allowing an indirect determination of the RMR. Although there was a reduction in the RMR in animals subjected to energy restriction, there was no increase in body weight, indicating that low quantity of ingested energy also reflected in low quantity of ingested energy to ensure that the basal activities and low energy were made available to be stored in the adipose tissue. Earlier, a reduced basal metabolic rate has been observed to be associated with caloric restriction; however, it is emphasized that the effect being 369 controversial since it could be related to weight loss and metabolic adaptation, especially of muscle and adipose tissues35. The weight loss is a major symptom of caloric restriction that can result from either excessive oxidative degradation of proteins or high lipolysis. These are related to the metabolic conditions in energy restricted animals, which demonstrate increased mobilisation of the triacylglycerols stored in adipose tissue (lipolysis). Moreover, structural proteins can be degraded, especially muscle. Thus, the carbon skeleton of amino acids can either be used to generate power or directed toward gluconeogenesis36, which maintains metabolic homeostasis during starvation and is associated with a broad spectrum of metabolic changes in fasting state. Thus, the reduction of cardiac proteins observed in G3 rats could be due to the degradation of these proteins, which supply carbon to maintain gluconeogenesis37. In addition, the lowest concentration of proteins in the cardiac tissue may be related to low protein synthesis, even though the level of serum protein did not change in animals submitted to energy restriction (Table 2). Energy restricted animals (30% and 60%) had the same heart-to-body weight ratio as the control group, indicating that cardiac mass decreased proportionally with body weight. This suggested that the impact of energy restriction on the heart did not involve substantial anatomical remodeling. Studies on the effect of caloric restriction on ROS levels have shown controversial results38. The data presented in this study showed that energy restriction decreased oxidative stress in cardiac tissue, as observed by decreased tissue level of HP, a product of lipid peroxidation. Previous studies39,40 have demonstrated low levels of ROS generation in rodents with a hypoenergetic diet. This was consistent with our observation of reduced myocardial HP level in animals subjected to a restrictive diet, since HP acts as a biomarker of oxidative stress. Furthermore, in another study, in dietary restriction, anti-aging and reduction in chronic disease with low formation of free radicals have been observed41. Moreover, the diet with caloric restriction is reported to increase the expression of proteins with cytoprotective properties, especially in cardiac tissue by increasing the cell resistance due to oxidative stress caused by free radicals42. The antioxidant system comprises SOD, which converts superoxide radicals into hydrogen peroxide (H2O2) and is the first line of defence against the 370 INDIAN J. BIOCHEM. BIOPHYS., VOL. 51, OCTOBER 2014 action of ROS and two enzymes — catalase and glutathione peroxidase (GSH-Px) that convert the H2O2 formed by SOD into H2O21. Since these enzymes remove H2O2, there is a compensatory relationship between the two to prevent the toxic effects of H2O243. In addition, GSH-Px protects against oxidative damage more than SOD, whose catalytic activity leads to an increase in H2O2. The imbalance in the oxidant/antioxidant system was clearly seen in the G3 group, since there was a decrease in the activity of myocardial HP. Although electron transport and oxidative phosphorylation are coupled and highly efficient biochemical processes in which O2 is reduced to H2O after receiving four electrons and 4H+, there is a potential that the mitochondrial generation of ROS may increase44. Recently, it is reported that transport of mitochondrial electrons acts as an important source of O2- in cardiac tissue during failure due to alterations in enzymatic complexes, leading to obstructed normal electron flow12. Glutathione (reduced-oxidized) system plays a key role in maintaining the balance between physiological pro-oxidants and antioxidants, which are essential for cell survival and \death45. In the present study, there was a decrease in myocardial GSH-Px activity in animals subjected to energy restriction of 60%, while catalase and SOD activities showed significant reduction in cardiac tissue in rats with energy restriction (30 and 60%). Since the myocardium depends almost exclusively on the energy released from the mitochondrial oxidation of fatty acids, oxidative metabolism contributes to increased mitochondrial ROS production. Thus, the low activity of antioxidant enzymes might be due to the lower ROS production, since energy restricted animals showed decreased oxidation of fatty acids. Our results agreed with the earlier reports38,46,47, wherein it is reported that caloric restriction reduces ROS production, thus maximizing longevity. It is demonstrated that caloric restriction with reduces energy metabolism and consequently a lower flow of electrons in the electron transport chain35. The decreased catalase activity as observed in the current study might be due to low consumption of oxygen. Several observations suggest that changes in the catalase activity depend on the consumption of oxygen48. It is demonstrated that catalase and SOD activities are linearly associated with VO2max in the skeletal muscle of individuals with a high aerobic capacity49. It is also observed that the rate of oxygen consumption and SOD and catalase activities declines with age in Musca domestica adults50. The increase in oxygen consumption might accelerate mitochondrial electron transport through the respiratory chain, resulting in leakage, leading to the formation of superoxide anions51. Since NADH and FADH2 are sources of electrons for the mitochondrial electron transport chain, decrease in both the lipid oxidation and VO2 in our energy restricted animals could have been due to the lower formation of O2-, thus regulating oxidative stress. In conclusion, the study demonstrated that energy restriction decreased the oxidation of carbohydrate and lipid and reduced energy expenditure, as revealed by the changes in the calorimetric parameters. Caloric restriction acted against oxidative stress in cardiac tissue, since lipid hydroperoxide levels were decreased in the tissue. Acknowledgements This research was supported by grants from CAPES (“Coordenação de Aperfeiçoamento de Pessoal de Nível Superior”). All authors of the manuscript cooperated throughout the development of the work (laboratory and experimental). References 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Jeckel-Neto E A, Ito Y, Sato T & Tauchi H (1994) Arch Gerontol Geriatric 19, 117-122 Masoro E J (2000) Exp Gerontol 35, 299-305 Genaro P S, Sarkis K S & Martini L A (2009) Arq Bras Endocrinol Metab 53, 667-72 McCay C M, Crowel M F & Maynard L A (1935) J Nutr 10, 63-79 Weindruch R & Walford R L (1982) Sci 15, 1415-1418 Wanagat J, Allison D B & Weindruch R (1999) Toxicol Sci 52, 35-40 Santos-Pinto F N, Luiz J & Griggio M A (2001) Int J Food Sci Nutr 52, 193-200 Valle A, Cralà-Niell A, Colom B, García-Palmer F J, Oliver J & Roca P (2005) Am J Physiol Endocrinol Metab 289, 15-22 Anson R M, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram D K, Lane M A & Mattson M P (2003) Proc Natl Acad Sci (USA) 100, 6216-6220 Marquezi M L & Costa A S (2008) Rev Mac 7, 119-129 Boudina S & Abel E D (2006) Physiol 21, 250-258 Tsutsui H, Kinugawa S & Matsushina S (2011) Am J Heart Circ Physiol 301, 2181-2190 Yamagishi S I, Edelstein D, DU X L, Kaneda Y, Guzman M & Brownlee M (2001) J Biol Chem 276, 25096-25100 Schauwen P & Hesselink M K C (2004) Diabetes 53, 1412-1417 Nojiri H, Shimizu T & Funakoshi M (2006) J Biol Chem 28, 33789-33801 KAWAHARA et al.: ENERGY RESTRICTION IMPACT IN CARDIAC TISSUE 16 How O J, Assum E, Severson D L, Chan W Y A, Essop M F & Larsen T S (2006) Diabetes 55, 466-473 17 Lowry O H, Rosebrough N L, Farr A L & Randall R J (1951) J Biol Chem 193, 265-275 18 Jiang, Z Y, Woolard A C S & Wolf S P (1991) Lipids 24, 861-869 19 Nakamura M, Hojoda S & Hayashi K (1974) Biochim Biophys Acta 358, 251-261 20 Ewing J F & Janero D R (1995) Anal Biochem 232, 243-248 21 Abuja P & Albertini R (2001) Clin Chim Acta 306, 1-17 22 Burneiko R C M, Diniz Y S, Galhardi C M, Rodrigues H G, Ebaid G M X, Faine L A, Padovani C R, Cicogna A C & Novelli E L B (2006) Food Chem Toxicol 44, 1167-1172 23 Ebaid G M X, Faine L A, Diniz Y S, Rodrigues H G, Gualhardi C M, Ribas B O, Fernandes A A H & Novelli E L B (2006) Food Chem Toxicol 44, 293-299 24 Strohl K P, Thomas A J, Jean P S, Schlenker E H, Koletsky R J & Schork N J (1997) J Appl Physiol 82, 317-323 25 Zar J H (1996) Bioestatistical analysis, 4th edn., Prentice Hall, New Jersey 26 Diniz Y S, Burneiki M R, Seiva F R F, Almeida F Q A, Galhardi C M, Filho J L V B, Mani F & Novelli E L B (2008) Intern J Cardiol 124, 98-99 27 Brito H F V (2004). Dissertação (Mestrado em Ciências Veterinárias) - Universidade Federal do Paraná; Curitiba, Paraná 28 Voet D, Voet J G & Pratt C W (2000) Fundamentos de Bioquímica, pp. 931, Porto Alegre. Editora Artmed 29 Labayen I, Forga L & Martinez J A (1999) Eur J Nutr 38, 158-166 30 Warhlich V & Dos Anjos L A (2001) Cad Saúde Pública 17, 801-817 31 Schneider P & Meyer F (2005) Rev Bras Med Esp 11, 193-196 32 Souza A, Ebaid G X, Seiva F R F, Rocha K H R, Galhardi C M, Mani F & Novelli E L B (2008) Evid Based Complem Altern 3, 1-7 [ 371 33 Mc Ardle W D, Katch F I & Katch V L (2011) Fisiologia do exercício: Nutrição, Energia e Desempenho Humano, 7th edn., Editora Guanabara Koogan, Rio de Janeiro 34 Novelli E L B, Souza G A, Ebaid G M X, Rocha K K H R, Mani F, Campos K E & Sforcin J M (2009) Obesity 18, 1754-1761 35 Redman L M, Heilbronn L K, Martin C K, Jonge L, Williamson D A, Delany & Ravussin (2009) PLoS ONE 4, 1-9 36 Orsolic N (2011) Eur J Pharmacol 656, 110-118 37 Gredilla R & Barja G (2005) Endocrinology 146, 3713-3717 38 Sohal R S, Agarwa L S, Candas M, Forster M J & Lal H (1994) Mech Ageing Dev 76, 215-224 39 Lopez-Torres M, Gredilla R, Sanz A & Barja G (2002) Free Radic Biol Med 32, 882-889 40 Weindruch E & Sohal RS (1997) N Engl J Med 337, 986-994 41 Lee C K, Allison D B & Brand J (2002) Proc Natl Acad Sci (USA) 99, 1493-1498 42 Velthuis-te Wierik E J, Van Den Berg H, Weststrate J A, Hof K H & Graaf C (1996) Eur J Clin Nutr 50, 214-219 43 Fleming J E, Miquel J & Cottrell S F (1982) Gerontology 28, 44-53 44 Bliska A, Kryczky A & Woldek L (2007) Postepy Hig Med Dows 61, 438-453 45 Lopez-Torres M, Gredilla R & Barja A S (2002) Free Radic Biol Med 32, 882-889 46 Qui X, Brown K & Hirsche Y (2010) Cell Metab 12, 662-667 47 Vicent H K & Taylor A G (2006) Int J Obes 30, 400-408 48 Jenkins R R, Friedland R & Howald H (1984) Int J Sport Med 5, 11-14 49 Sohal R S, Farmer K L, Allen R G & Cohen N R (1984) Mech Aging Dev 24, 185-195 50 Vicent H K, Powers S K & Diks A J (2001) Int J Obes 25, 378-388 51 Sarkhail P, Rahmanipour S, Fadyevatan S, Mohammadirad A, Dehghan G, Amin G, Shafiee A & Abdoliahi M (2007) Pharmacol Res 56, 261-266
Baixar