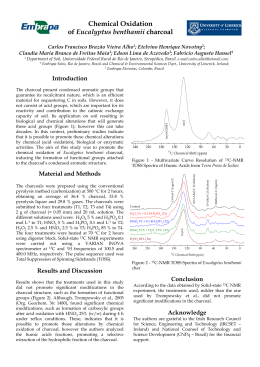
Synthesis of Co catalysts supported on carbon nanotubes and their application in the catalytic reforming of ethanol for hydrogen production 1 2 3 Estevão F. Mai , Fábio B. Noronha , Cristiane B. Rodella , and Victor Teixeira da Silva1* 1 Federal University of Rio de Janeiro / COPPE / Chemical Engineering Program / NUCAT, P.O. Box 68502, Rio de Janeiro, RJ 21945-970, Brazil 2 Instituto Nacional de Tecnologia, Av. Venezuela 82, CEP 20081-312, Rio de Janeiro, Brazil 3 Laboratório Nacional de Luz Síncrotron (LNLS), C. P. 6192, 13083-970, Campinas, SP, Brazil *[email protected] Introduction Co-based catalysts have been extensively studied for the steam reforming of ethanol (SR) [1,2], because it is a low-cost alternative to noble metals. In spite of the high activity of Co-based catalysts, their stability is still a challenge. Different approaches such as the increase of the steam/ethanol molar ratio of the feed, the addition of oxygen to the feed, support modification and the control of ensemble size have been proposed to prevent carbon formation. The control of ensemble size aims to inhibit carbon formation by hindering the mechanistic pathway for carbon formation during ethanol steam reforming and is an approach that is essentially identical to the one used for hindering coke formation during methane steam reforming. Ensemble size control may be achieved by varying the number of superficial groups on carbon nanotubes (CNT) available for metal deposition. The aim of this work was to study the effect of different oxidative treatments on carbon nanotubes to use them as a support for Co-based catalysts and evaluate their performance on the SR and oxidative steam reforming (OSR) of ethanol for hydrogen production.. Materials and Methods Commercial carbon nanotubes (NANOCYLTM NC3150) were treated with two different oxidants: HNO3 or H2O2. In the treatment with H2O2 (30% v/v) the CNT were kept in contact with a hydrogen peroxide solution for 96 hours under stirring at room temperature (NTC_H2O2). In the treatment with HNO3 (65% v/v), the CNT were stirred for 6 hours at 363K under reflux of nitric acid. (NTC_HNO3). After this step, the materials were washed with deionized water, filtered and dried at 383K. A load of 1wt. % Co was incorporated by impregnation of the oxidized CNT using an aqueous solution of Co(NO3).6H2O. After impregnation, the samples (1%Co/CNT, 1%Co/CNT_H2O2 and 1%Co/CNT_HNO3) were dried at 373 K and subsequently reduced with 30% (v/v) H2 / He (100 mL min-1) at 623K, followed by passivation with a mixture containing 0.5% (v/v) O2/N2 (50 mL min-1). The catalysts were characterized by N2 physisorption, Fourier Transformed Infrared spectroscopy (FTIR), Raman spectroscopy, X-Ray Diffraction (XDR), X-Ray Photoelectronic Spectroscopy (XPS) and TPD of adsorbed ethanol. SR of ethanol was performed in a fixed bed reactor at atmospheric pressure. Prior to reaction, the catalysts were reduced at 773 K for 1 h. The reactions were carried out at 773 K and W/Q = 0.02 g.s/cm3. A H2O/ethanol molar ratios of 3.0 or 10.0 were employed. Results and Discussion Figure 1 shows the ethanol conversion and product distribution as a function of time on stream (TOS) obtained for Co/NTC and Co/NTC_HNO3 during SR. Co/NTC exhibited a higher initial ethanol conversion than that of Co/NTC_HNO3 or Co/NTC_H2O2 (not shown). However, all catalysts underwent significant deactivation during TOS. For the Co/NTC catalyst, H2 and acetaldehyde were the main products formed, indicating that ethanol dehydrogenation was the reaction occurring. For the Co-based catalysts over the functionalized carbon nanotubes, H2 and CO2 were formed, which suggests that SR of ethanol is the main reaction pathway occurring. Acetaldehyde formation significantly decreased when the supports were previously treated with HNO3 and H2O2 with the former presenting a better performance than the later. Besides that, XPS data suggests that chemical treatments with either H2O2 or HNO3 were effective in improving cobalt dispersion. (a) (b) Figure 1. Product distribution and ethanol conversion using 1%Co/CNT (a)and 1%Co/CNT_HNO3 (b). The association between the characterization results and catalytic activity allow to conclude that the functionalization of the CNTs and de the type of oxidant used has a strong influence on the performance of the catalysts. Significance Chemical treatments of carbon nanotubes with either H2O2 or HNO3 lead to an improvement in cobalt dispersion and therefore to more selective catalysts towards hydrogen production during ethanol steam reforming. References 1. R. Padilla, M. Benito, L. Rodríguez, A. Serrano, G. Muñoz, L. Daza, Int J Hydrogen Energy, 35 (2010), 8921-8928. 2. P.K. Seelam, M. Huuhtanem, A. Sápi, M. Szabó, K. Kordás, E. Turpeinen, G. Tóth, R.L. Keiski Int J Hydrogen Energy,35 (2010), 12588-12595.
Baixar