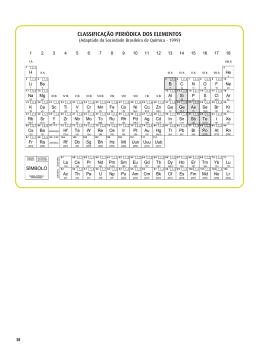
ROTEIRO DE RECUPERAÇÃO – CIÊNCIAS Professora da Disciplina: Yara Tappis Aluno (a): 9º ano --- Ensino ______ Período: Matutino - FUNÇÕES QUÍMICAS - TABELA PERIÓDICA - LIGAÇÕES QUÍMICAS - ELETRÓLITOS - INDICADORES -SEMELHANÇAS ATÔMICAS - MASSA MOLECULAR 1) Observe a lista de substâncias químicas abaixo: Ba ( OH ) 2 CaCO 3 NaOH SO 2 H4 P 2 O 7 CO 2 MnO 4 11 /15 Nº: CONTEÚDO: H2 S O 4 Data: / Valor da avaliação: 3º TRIMESTRE Nota: HBr H2O2 H 3 PO4 Na NO3 HI Extraia da lista: a) Um monoácido ___________________________________________________________________ b) Uma base _______________________________________________________________________ c) Uma substância binária _______________________________________________________________ d) Um poliácido ____________________________________________________________________ e) Uma dibase _____________________________________________________________________ f) Um oxisal _______________________________________________________________________ g) Um hidrácido ____________________________________________________________________ h) UM poliácido ____________________________________________________________________ i) Um óxido _______________________________________________________________________ j) Uma substância ternária ___________________________________________________________ k) Um ácido binário _________________________________________________________________ l) Um oxiácido _____________________________________________________________________ m) Um dióxido ______________________________________________________________________ 2) Explique o que são indicadores e determine exemplos utilizados em laboratório. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 3) Escreva sobre a estrutura da tabela periódica do elementos. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 4) Explique o que são eletrólitos. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 5) Defina ácidos , bases , sais e óxidos. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 6) Cite formas de identificar as moléculas de ácidos, bases, sais e óxidos. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 7) Diferencie ligações químicas iônicas de ligações químicas covalentes. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 8) De que forma surgem os cátions e os ânions? _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ ________________________________________________________________________________________________ 9) Simbolize quimicamente a ligação química entre o potássio e o cloro. 10 ) Explique o que é uma reação química de neutralização. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 11) Explique o que são ISÓTOPOS, ISÓBAROS E ISÓTONOS E DÊ EXEMPLOS. _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ _________________________________________________________________________________________________ 12 ) Use sua tabela periódica e calcule as massas moleculares de todos os compostos químicos propostos no exercício 1. _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ _____________________________________________________________________________________________________ __________________________________________________________________________________________________
Download