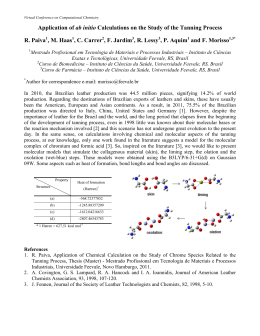
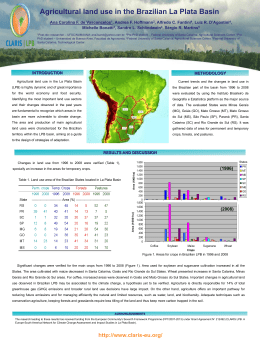
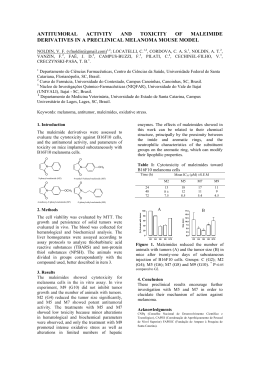
57º Congresso Brasileiro de Genética Resumos do 57º Congresso Brasileiro de Genética • 30 de agosto a 2 de setembro de 2011 Centro de Convenções do Hotel Monte Real Resort • Águas de Lindóia • SP • Brasil www.sbg.org.br - ISBN 978-85-89109-06-2 18 Evaluation of teratogenicity, mutagenicity and phagocytic activity of Gochnatia polymorpha ssp. floccosa in pregnant mice David, N1; Mauro, MO2; Cunha-Laura, AL3; Monreal, MTDF3; Monreal, ACD3; Kassuya, CAL4; Stefanello, MEA5; Oliveira, RJ1. 1 Coordenadoria de Educação Aberta e a Distância, Universidade Federal de Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brasil; 2Programa de Pós-graduação em Ciências Biológicas (Biologia Celular e Molecular) do Instituto de Biociências de Rio Claro, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Rio Claro, São Paulo, Brasil; 3Centro de Ciências Biológicas e da Saúde, Universidade Federal de Mato Grosso do Sul, Campo Grande, Mato Grosso do Sul, Brasil; 4 Faculdade de Ciências da Saúde, Universidade Federal da Grande Dourados, Dourados, Mato Grosso do Sul, Brasil; 5 Departamento de Química, Universidade Federal do Paraná, Curitiba, Paraná, Brasil. [email protected] Keywords: Cambará, Asteraceae, Micronucleus, Phagocytosis, In vivo Gochnatia polymorpha ssp. floccosa, known in Brazil as cambará is used in folk medicine, usually in the form of teas made from its flowers, leaves and bark, for the treatment of inflammation and respiratory tract infections. This study aimed to evaluate the teratogenicity, mutagenicity and phagocytic activity of ethanol extract of G. polymorpha (EEGP) in pregnant mice. We used 15 pregnant Swiss mice were subdivided into three groups: (I) Control - received hydroalcoholic solution (1.2%), Oral - gavage (po) throughout the gestational period, (II) Organogenesis - received a solution hydroalcoholic EEGP the concentration of 100mg/kg body weight (bw, po) from 5th to 15th day of gestation (dg), (III), Gestational - received water-alcohol solution at a concentration of 100mg/kg EEGP (pc, po) throughout the gestational period. At 16, 17 and 18 dg samples were collected from peripheral blood to perform the micronucleus assay (staining with acridine orange - 1mg/mL). In the 18th dg females were euthanized to collect and fetuses and spleens. The fetuses were fixed for later analysis and spleens, homogenized and an aliquot (50μL) was placed on slides previously stained with acridine orange (1mg/mL) for analysis of splenic phagocytosis. Data were evaluated by ANOVA and indicated that: (i) the extract at the dose tested and protocol, it has no teratogenic activity (external review), (II) has no mutagenic activity, and (III) does not modify the phagocytic activity . Preliminary data indicate that none of teratogenicity fetus showed external abnormalities. The mutagenicity data show that the mean micronuclei was 0.20 ± 0.20, 1.00 ± 0.45 and 0.40 ± 0.25 for the 16 dg, 0.40 ± 0.25, 0.60 ± 0.55 and 0.60 ± 0.40 for the 17 th dg, 0.20 ± 0.20, 0.20 ± 0.20 and 0.60 ± 0.25 for 18 of dg to the control groups, organogenesis and gestational , respectively. The analysis showed evidence of splenic phagocytosis 34.00 ± 2.34, 29.20 ± 2.37 and 39.20 ± 3.02 for control groups, organogenesis and pregnancy, respectively. Since this plant is widely used by the population, including pregnant women, the data suggest safety in the use thereof. However, further studies are needed since it is not possible to extrapolate directly from experimental data from animals to humans. Financial Support: Pró-reitoria de Pesquisa e Pós Graduação (PROPP/UFMS); Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT)
Download