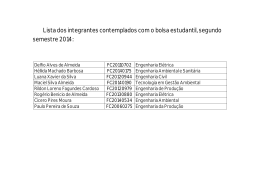
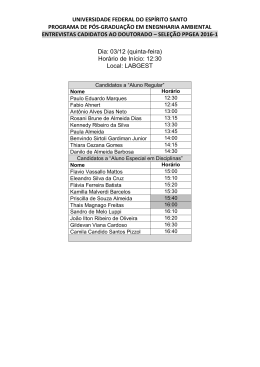
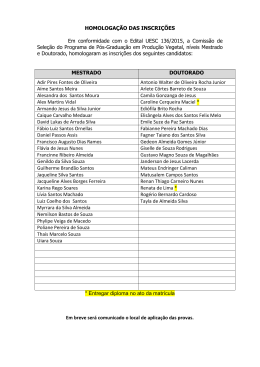
CURRICULUM VITÆ 1. IDENTIFICATION Name: Almeida, António José Leitão das Neves. Place of Birth: Moçâmedes - Angola. Date of Birth: 16th May 1963. Address: Unidade de Ciências e Tecnologia Farmacêuticas Faculdade de Farmácia Universidade de Lisboa Av. Prof. Gama Pinto 1649-003 Lisboa Tel. +351 21 7946409 Fax. +351 21 7937703 E-mail: [email protected] EDUCATION 2.1. Higher Education 1987 - Licenciatura in Pharmaceutical Sciences (Industrial Pharmacy), University of Lisbon, Faculty of Pharmacy, Portugal. 1993 - Ph.D., The University of Aston, Department of Pharmaceutical and Biological Sciences, Birmingham, UK. 2005 - Habilitation (Agregação) in Pharmaceutical Sciences, University of Lisbon, Faculty of Pharmacy, Portugal. 2.2. Qualifications 1987 - Qualified pharmacist, by the Portuguese Pharmaceutical Society (Ordem dos Farmacêuticos), Portugal. 3. PROFESSIONAL ACTIVITY 3.1. ACADEMIC CARRIER 1986/88 - Monitor (demonstrator), University of Lisbon, Faculty of Pharmacy, Portugal. 1988/92 - Junior Lecturer Assistant, University of Lisbon, Faculty of Pharmacy, Portugal. 1991/93 - Demonstrator, Department of Pharmaceutical and Biological Sciences, Aston University, Birmingham, UK. 1992/94 - Senior Lecturer Assistant, University of Lisbon, Faculty of Pharmacy, Portugal. 1994/01- Assistant Professor, University of Lisbon, Faculty of Pharmacy, Portugal. 1994/97 - Visiting Associate Professor, Instituto Superior de Ciências da Saúde - Sul. 2001/ - Associate Professor, University of Lisbon, Faculty of Pharmacy, Portugal. 2007 – Visiting Professor, University of Navarra, Faculty of Pharmacy, Spain. 1 3.2. TEACHING ACTIVITY 3.2.1. Under-gradate level 1986/88 - Genetic Engineering and Pharmaceutical Biotechnology, University of Lisbon, Faculty of Pharmacy, Portugal. 1988/.. - Pharmaceutics and Pharmaceutical Technology, University of Lisbon, Faculty of Pharmacy, Portugal. Teaching has included: pharmaceutical compounding, parenteral dosage forms, disperse systems, topical dosage forms, sterilization, lyophilisation, drug delivery systems, drug targeting, development pharmaceutics, stability of drugs, regulatory affairs and quality assurance. 3.2.2. Post graduate level 1994/.. - Master course in Advanced Pharmaceutics, University of Lisbon, Faculty of Pharmacy, Portugal, teaching controlled drug delivery, drug delivery systems and pharmaceutical development. 1995 - 1st European Course on New Forms and New Routes of Administration of Drugs, University of Coimbra, Faculty of Pharmacy, Portugal, teaching vaccine delivery systems. 1997/.. - Master course in Community Pharmacy, University of Lisbon, Faculty of Pharmacy, Portugal, teaching advanced pharmaceutical compounding. 1998/00 - Post-graduation course on Pharmaceutical Medicine, New University of Lisbon, Faculty of Medical Sciences, Portugal, teaching pharmaceutical development and stability of drugs. 1999/- Master Couse on Advanced Biopharmaceutics and Pharmacokinetics, University of Lisbon, Faculty of Pharmacy, teaching Controlled drug delivery and drug delivery systems. 2000 - 6th European Course on New Forms and New Routes of Administration of Drugs, University of Santiago de Compostela, Faculty of Pharmacy, Spain, teaching drug delivery to mucosal surfaces. 2000 - Master course on Medicines Technology, University of Coimbra, Faculty of Pharmacy, Portugal, teaching targeting of particulate systems to mucosal surfaces. 2001 - Master course on Pharmaceutical Technology, University of Oporto, Faculty of Pharmacy, Portugal, targeting of particulate systems to mucosal surfaces. 2001/.. - Post-graduation course in Economical Evaluation of Medicines, Technical University of Lisbon, School of Economics, Portugal, teaching pharmaceutical development and stability of drugs. 2001/ - Master course in Pharmaceutical Regulatory Affairs, University of Lisbon, Faculty of Pharmacy, Portugal, being responsible for the subject Evaluation of Quality. 2 3.3. SCIENTIFIC ACTIVITY 1986/87 - Research student, University of Lisbon, Faculty of Pharmacy, Portugal. Project: Modification of Streptomyces erythreus strains to improve streptomicin production. Supervisor: Prof. Dr. Rui Vidal Correia da Silva. 1987/88 - Research student, Instituto Gulbenkian de Ciência, Oeiras. Project: Caracterization of Bacillus subtilis mutants. Supervisor: Prof. Dr. Hermínia de Lencastre. 1990/93 - PhD research student, Department of Pharmaceutical and Biological Sciences, Aston University, Birmingham, UK. Project: Particulate carriers as immunological adjuvants. Supervisor: Prof. Dr. H. Oya Alpar. 1994/ - Scientist at the Unidade de Ciências e Tecnologia Farmacêuticas (UCTF), University of Lisbon, Faculty of Pharmacy, Portugal, responsible for a research line on particulate carrier systems. 1995/96 - Visiting scientist at the Freie Universität Berlin, Institut für Pharmazie, Berlim, Germany, (Prof. Dr. Rainer H. Müller), working on the production and characterisation of solid lipid nanoparticles. 3.3.1. Research Projects I. Development of new dosage forms for the controlled delivery of drugs. Funded by the pharmaceutical industry (Tecnimede - Sociedade Técnico Medicinal, S.A.) and the European Union (PEDIP II). Duration: 1995-1997. II. Development of an antioxidant solution for oral administration. Funded by the pharmaceutical industry (Helsinn-Produtos Farmacêuticos, S.A). Duration: 1995-1999 III. Production of solid lipid nanoparticles containing protein antigens. In collaboration with the Freie Universität Berlin, Institut für Pharmazie, Germany (Prof. Dr. Rainer H. Müller). Funded by DAAD (Germany) and INVOTAN (Portugal). Duration: 1995-2002. IV. Production and characterisation microencapsulated vaccines for oral or nasal administration (P/P/S5, IMUNOPOR). R&D project funded by the European Union (SINDEPEDIP), in collaboration with several institutions: Laboratório Sorológico, Laboratório Medinfar, IBET, INETI, IHMT/CMDT e Laboratório de Microbiologia da FFUL. Coordinator of P/P/S5 (Development of a vaccine agains strangles). Duration: 19972000. V. Carrier systems for diagnosis and therapeutic applications associated with the lung lymphatics. Funded by the Ministry of Science (Praxis XXI, Ciências da Saúde, 2/2.1/SAU/1305/95), in collaboration with IBILI (Coimbra) and Faculdade de Farmácia, Universidade de Coimbra. Duration 1997-2000. VI. Biological evaluation of new controlled release dosage forms for beta-adrenergic blocking drugs. Funded by the Ministry of Science (Praxis XXI, Ciências da Saúde, 2/2.1/SAU/1418/95). Responsible scientist: Prof. J.A.G. Morais. Duration 1998-2003. VII. Production and release properties of microencapsulated drugs using supercritical fluids. Funded by the Ministry of Science (POCTI/1999/EQU/34961). In collaboration with Instituto Superior Técnico, Lisboa. Responsible scientist: Prof. E. Gomes de Azevedo. Duration 2001-2004. 3 VIII. Therapeutic systems for tuberculosis treatment. Funded by the Ministry of Science (POCTI/Sapiens/36416/99). In collaboration with UNFAB/ INETI, Lisboa Responsible scientist Dr Eugénia Cruz. Started January 2002. IX. Development of a microparticulate system for colonic drug delivery. Funded by the Acções Integradas Luso-Espanholas (Acção nº E-15/03). In collaboration with the Facultad de Farmácia, Universidad de Sevilla, Spain (Prof. Mercedes Fernández Arévalo). X. Development of polymeric microspheres for the delivery of Streptococcus equi antigens. Project leading to the PhD thesis of Helena Isabel Fialho Florindo (FCT-SFRHBD/14370/03), in collaboration with the Centre for Drug Delivery Research, London School of Pharmacy, Reino Unido (Prof. H. O. Alpar). Duration: 2003-2007. XI. A new approach to the production of an anti streptococcal vaccine. Funded by the Ministry of Science (POCTI/BIO/59147/2004). In collaboration with IBET (Portugal), CDDR, London and the Karolinska Institute, Sweden. Duração: 2005-2008. XII. Protein Microencapsulation Using Supercritical Fluids. Financiado pela FCT (POCTI/EQU/55911/2004). In collaboration with the Instituto Superior Técnico, Lisboa. Responsible scientist: Prof. E. Gomes de Azevedo. Duration 2005-2008. 3.3. OTHER ACTIVITIES AT THE UNIVERSITY OF LISBON 1998/02 - President of the Faculty’s Assembly of Representatives. 2003/.. - Vice-President of the Faculty’s Scientific Board. 3.5. 1996/ - 3.6. MEDICINE EVALUATION Quality expert of the Portuguese Medicines Agency (INFARMED) and the European Medicines Agency (EMEA), for new medicines evaluation, having participated in the evaluation of many new medicines by the national, mutual recognition and centralised procedures for the, including the following: Azilect® (rasagiline mesilate), Carbaglu® (carglumic acid), Circadin® (melatonine), Fuzeon® (enfuvirtide), Galsulfase® (ethyl (recombinant human N-acetylgalactosamine-4-sulfatase), Lyxia® eicosapentenoate), Nexavar® (sorafenib), Orathecin® (rubitecane), Oxytrol® (oxibutinine), Sustiva®/Stocrin® (efavirenz), Xaprila® (xaliproden hydrochloride). OTHER ACTIVITIES 1988/89 - Regulatory affairs at Merck Sharp & Dohme Lda./MSD-AGVET, Lisbon. 1988/89 - National Service (Portuguese Army), at the Military Laboratory, as a Second Lieutenent. 1997/ - Referee of Acta Biomaterialia, Drug Delivery Science and Technology, Drug Development and Industrial Pharmacy, European Journal of Pharmaceutics and Biopharmaceutics, Journal of Applied Polymer Science, Journal of Controlled Release, Pharmaceutical Research e Vaccine. 4 1997/ - Vice-President of the CRS Spanish-Portuguese Local Chapter of the Controlled Release Society (SPLC-CRS) (1998-2002); Treasurer (1997/98); Secretary (20022005). 4. PUBLICATIONS 4.1. THESES AND REPORTS 1. Almeida, A.J. (1987). Melhoramento de estirpes de Streptomyces erythreus, via engenharia genética para a produção de eritromicina. Final dissertation of the first degree in Pharmaceutical Sciences. University of Lisbon, Faculty of Pharmacy. 2. Almeida, A.J. (1988). Contribuição para a caracterização de mutantes de Bacillus subtilis resistentes à infecção pelo fago Φ105. Final dissertation of the postgraduation course Modern Techniques in Biology and Biotechnology, Instituto Gulbenkian de Ciência, Oeiras. 3. Almeida, A.J. (1993). Particulate carriers as immunological adjuvants. Ph.D. thesis, Department of Pharmaceutical and Biological Sciences, The University of Aston in Birmingham, Reino Unido. 4. Almeida, A.J. (2005). Micro- and nanoparticulate translocation across epithelial surfaces: mechanisms and therapeutic applications. Scientific lecture presented for habilitation, University of Lisbon, Faculty of Pharmacy. 4.2. SEMINARS 1. Almeida, A.J. (1989). Alguns aspectos da aplicação da biotecnologia à industria farmacêutica. Reuniões Temáticas do Laboratório Militar de Produtos Químicos Farmacêuticos, Lisbon, 17th March. 2. Almeida, A.J. (1995). Microsferas biodegradáveis como vectores de antigénios. Conferência proferida nos II Encontros Farmacêuticos do Laboratório Militar de Produtos Químicos e Farmacêuticos, Lisboa, 25th January. 3. Almeida, A.J. (1996). Biodegradable particulate carriers as potential vaccine delivery systems. Seminar at the Franz-Volhard-Klinik, Medizinische Fakultät der HumboldtUniversität zu Berlin, Germany, 20th March. 4. Almeida, A.J. (1997). Trends in nasal delivery of vaccines using particulate carriers. Seminar at the Faculdad de Farmacia, Universidad de Santiago de Compostela, Spain, 17th April. 5. Almeida, A.J. (1997). Utilización de micropartículas en inmunización mucosal. Seminar at the Centro de Investigación - Pierre Fabre Ibérica, S.A., Barcelona, Spain, 24th July. 6. Almeida, A.J. (2000). Fórmulas oficinais e magistrais: requisitos de qualidade. 3º Encontro de Orientadores de Estágio de Licenciatura da FFUL. Lisbon, 8th Abril. 7. Almeida, A.J. (2004). Drug delivery and targeting using lipid nanoparticles. Seminar at the Faculdad de Farmacia, Universidad de Santiago de Compostela, Spain, 22nd March. 8. Almeida, A.J. (2005). Lipid nanoparticles for controlled drug delivery. Conferência proferida na Freie Universität Berlin, Institut für Pharmazie, Berlin, Germany, 18th November. 5 4.3. PRESENTATIONS AT SCIENTIFIC MEETINGS 4.3.1. Plenary Lectures and Invited Oral Presentations 1. Almeida, A.J. (1996). Aplicações farmacêuticas de vectores coloidais. II Jornadas Farmacêuticas do Instituto Superior de Ciências da Saúde Sul, Monte da Caparica, Portugal, 15-16 May. 2. Almeida, A.J. (1997). Sistemas avançados de veiculação de fármacos. Reunião Anual do Colégio de Indústria Farmacêutica da Ordem dos Farmacêuticos, Tomar, Portugal, 4-6 April. 3. Almeida, A.J. (1998). Mucosal delivery and absorption of particulate systems. Proceed. III Congresso Hispano-Luso de Libertação Controlada de Medicamentos (Lisboa, Portugal), pp. 9 (PLENARY LECTURE). 4. Almeida, A.J. (1999). Ensaios de estabilidade e prazo de validade. A Envolvente da Avaliação de Medicamentos, INFARMED, Lisbon, Portugal, 15th March. 5. Almeida, A.J. (1999). Mucosal delivery of particles - absorption and translocation accross epithelial surfaces. I Mediterranean CRS Conference: Drug Delivery in the Third Millennium (Pisa, Italy). Acta Tecnologiae et Legis Medicamenti, 10: 77 (PLENARY LECTURE). 6. Almeida, A.J. (1999). Absorção de vectores medicamentosos coloidais: factores condicionantes e aplicações terapêuticas. VI Fórum Farmacêutico, subordinado ao tema Novas Terapias e Tecnologias Farmacêuticas, Oporto, Portugal, 3-4 November. 7. Rodrigues, M., Peiriço, N., Almeida, A.J., Matos, H., Gomes de Azevedo, E. (2002). Supercritical fluid technology: applications to microparticle formation for controlled drug release. V Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Seville, Spain), Abstract book, pp.39-40. 8. Almeida, A.J. (2003). Comunidade científica e inovação em Portugal. Ciclo de Conferências: Indústria Farmacêutica e Medicamentos Inovadores, Ordem dos Farmacêuticos, Lisbon, 20th May. 9. Almeida, A.J., Toscano, C., Videira, M. (2004). Therapeutic applications of lipid nanoparticles administered by alternative routes. XII International Workshop on Bioencapsulation, Vitoria, Spain, 24-26 September (PLENARY LECTURE). 10. Almeida, A.J. (2004). Pulmonary delivered lipid nanoparticles as potential drug carriers for cancer therapy. 8th European Congress of Pharmaceutical Sciences (EUFEPS 2004), Brussels, Belgium. Eur. J. Pharm. Sci., 23 (Suppl. 1): S25-S26. 11. Almeida, A.J. (2004). Formulação magistral para necessidades hospitalares específicas. Workshop: Medicamentos para grupos de pacientes com necessidades hospitalares específicas - V Congresso da APFH, Lisbon, Portugal, 24 November. 12. Almeida, A.J. (2006). Ensaios de estabilidade aplicados ao produto final. Conferência proferida no Seminário Especializado: Stability Testing, Institute for International Research, PTI–Pharmaceutical Training Institute (Lisbon, Portugal), 14th February. 13. Almeida, A.J. (2006). Estudos de fotoestabilidade. Conferência proferida no Seminário Especializado: Stability Testing, Institute for International Research, PTI–Pharmaceutical Training Institute (Lisbon, Portugal), 14th February. 6 14. Almeida, AJ. (2006). Vaccination strategies using nanoparticles. Simpósium New Strategies for Administration of Drugs Sociedade Portuguesa de Ciências Farmacêuticas (SPCF), 17th May. 4.3.2. Oral Presentations 1. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1992). The efficient encapsulation of biologically intact high molecular weight proteins into submicron polylactide microspheres. 6th International Conference on Pharmarmaceutical Technology (Paris, France). Abstract book, pp. 39. 2. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1992). Protein adsorbed polylactide microspheres as mucosal immunoadjuvants. 6th International Conference on Pharmaceutical Technology (Paris, France). Abstract book, pp. 40. 3. Almeida, A.J. (1993). Poly(lactic acid) microspheres as immunological adjuvants for tetanus toxoid. Newpharm’93 (Loughborough, UK). 4. Poyner, E.A., Alpar, H.O., Almeida, A.J., Brown, M.R.W. (1993). Liposomal and microencapsulated tobramycin: optimisation, antibacterial activity and in vivo distribution. 9th International Symposium on Microencapsulation (Ankara, Turkey). Abstract book, pp. 85a. 5. Almeida, A.J., Runge, S., Müller, R.H. (1996). Production and characteristics of peptideloaded solid lipid nanoparticles (SLN). 42nd Annual Congress of the International Association for Pharmaceutical Technology (Mainz, Germany). Eur. J. Pharm. Biopharm., 42 (Suppl.): 44S. 6. Cerdeira, A.M., Brandão, A., Goucha, P., Cabral Marques, H.M., Almeida, A.J. (1998). Preparation and characterisation of beads containing a nabumetone: dimethyl-β-cyclodextrin complex. 17th Pharmaceutical Technology Conference (Dublin, Ireland). 7. Azevedo, A.F., Almeida, A.J. (1998). Methods for the recovery of an intact protein molecule from PLGA microspheres: a comparative study. III Congresso Hispano-Luso de Libertação Controlada de Medicamentos (Lisbon, Portugal). Abstract book, pp. 27-28. 8. Almeida, A.J, Almeida, S., Gonçalves, C., Videira, M., Azevedo, A.F. (2000) Evaluation of different methods for surface hydrofobicity determination in particulate colloidal carriers. IV Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Vitoria, Spain). Abstract book, pp.41-42. 9. Ferreira Almeida, P., Almeida A.J. (2001). Sustained release of pindolol from calcium alginate beads: production and characterisation. II Mediterranean CRS Meeting: New Perspectives in Controlled Release (Athens, Greece). Abstract book, pp.186. 10. Videira, M., Florindo, H., Almeida, A.J. (2002). Preparation of solid lipid nanoparticles (SLN): a potential protein delivery system. V Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Seville, Spain). Abstract book, pp.69-70. 11. Leandro, J., Nascimento, C., Tavares de Almeida, I, Almeida A.J., Leandro, P.(2005). Effect of mannitol and glycerol on storage stability of human phenylalanine hydroxylase. II Congress of the Portuguese Society of Pharmaceutical Sciences/VI Congress of the Portuguese-Spanish Chapter of the Controlled Release Society (Coimbra, Portugal). Rev. Port. Farm., 52 (Supl. 2): 7-8. 7 12. Florindo, H.F., Pandit, S., Somavarapu, S., Almeida, A.J., Alpar, H.O. (2005). Immune response induced by S. equi enzymatic extract adsorbed to surface modified polycaprolactone microspheres. Proc. Intern. Symp. Control. Rel. Bioact. Mater., 32: (Miami, EUA), pp. 805806. 13. Videira, M., Florindo, H.F., Gouveia, L.F. Lobato, M.R., Almeida, A.J. (2005). Triglyceryde nanoparticles as potential carriers for paclitaxel. 15th International Symposium on Microencapsulation (Parma, Italy). Abstract book, pp. 225-226. 14. Alpar H.O., Florindo, H.F., Pandit, S., Gonçalves, L.M.D., Somavarapu, S., Almeida, A.J. (2006). Production and immune response characterisation of S. equi enzymatic extract adsorbed polycaprolactone microshperes. 9th International Congress of the World Equine Veterinary Association (Morocco) 22-26 January 2006. 15. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). SLN and NLC as viscoelastic enhancers for topical drug delivery. XIV International Workshop on Bioencapsulation (Lausanne, Switzerland). 16. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2006). Leite materno e a presença de mercúrio. XXXIV Jornadas Nacionais de Pediatria, (Aveiro, Portugal). Abstract book, pp. 47. 17. Florindo, H.F., Gonçalves, L.M.D., Alpar, H.O., Almeida, A.J. (2006). Adsorption of Streptococcus equi enzymatic extract on the surface modified PCL nanospheres. VII Congress Portuguese-Spanish Congress on Controlled Drug Delivery (Pamplona, Spain). Abstract book, pp. 32-33. 18. Albuquerque, M., Silva, A., Salgado, A., Duarte, M.A., Almeida, A.J. (2006). Avaliação da estabilidade de uma fórmula magistral de pirazinamida em suspensão líquida oral para uso em pediatria. 11º Simpósio Nacional da APFH, (Estoril, Portugal). Abstract book, pp. 21. 19. Gonçalves, H., Mancini, G., Ribeiro, H.M., Silva, A, Duarte, M.A., Almeida, A.J. (2006). Desenvolvimento e caracterização reológica de uma fórmula magistral de L-arginina em gele semi-sólido para aplicação tópica. 11º Simpósio Nacional da APFH, (Estoril, Portugal). Abstract book, pp. 22. 20. Barros, C.T., Almeida, A.J. (2006). Perfil da formulação extemporânea de medicamentos orais para pediatria nos serviços farmacêuticos hospitalares. 11º Simpósio Nacional da APFH, (Estoril, Portugal). Abstract book, pp. 23. 4.3.3. Poster Communications 1. Lencastre, H., Estrela, A.I., Almeida, A.J. (1987). Characterization of a Bacillus subtilis mutant resistant to infection by phage Φ105. 8º Congresso Nacional de Bioquímica (Póvoa de Varzim, Portugal). Ciência Biológica, 12 (Supl.): 254. 2. Alpar, H.O., Rovamo, L., Devi, K., Almeida, A.J., Collier, P.J., Brown, M.R.W. (1991). In vitro evaluation of antibacterial activities of liposomal tobramycin against Pseudomonas aeruginosa. 128th British Pharmaceutical Conference (Liverpool, UK). J. Pharm. Pharmacol., 43 (Suppl.): 122P. 8 3. Almeida, A.J., Alpar, H.O., Williamson, D., Brown, M.R.W. (1992). Poly(lactic acid) microspheres as immunological adjuvants for orally delivered cholera toxin B subunit. 643rd Meeting of the Biochemical Society (Warwick, UK). Biochem. Soc. Trans., 20: 316S. 4. Alpar, H.O., Malik, A., Almeida, A.J., Brown, M.R.W. (1992). Preparation and characteristics of gentamicin-containing poly(lactide-co-glycolide) microspheres for lung targeting. 129th British Pharmaceutical Conference (Birmingham, UK). J. Pharm. Pharmacol., 44 (Suppl.): 1082. 5. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1993). Poly(L-lactic acid) microspheres as a vaccine adjuvant for tetanus toxoid. Science Link’93 (Macclesfield, UK). Abstract book, pp. 56A. 6. Almeida, A.J., Alpar, H.O. Brown, M.R.W. (1993). Formulation studies on particulate carriers as immunological adjuvants. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 20: 390-391 (Washington, USA). 7. Alpar, H.O., Almeida, A.J., Brown, M.R.W., Williamson, E.D. (1994). Immune responses to mucosally administered tetanus toxoid in biodegradable PLA microspheres. Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 21: 867-868 (Nice, France). 8. Almeida, A.J., Alpar, H.O. (1994). Microsphere adjuvanticity: influence of polymer, dose and size on the humoral response to microencapsulated tetanus toxoid. 2nd European Congress of Pharmaceutical Sciences (Berlin, Germany). Eur. J. Pharm. Sci., 2: 189. 9. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1996). Parameters affecting the formulation of NSAIDs-containing gastroresistant beads. 3rd European Congress of Pharmaceutical Sciences (Edimburgh, UK). Eur. J. Pharm. Sci., 4 (Suppl.): S157. 10. Almeida, A.J., Dingler, A., Runge, S., Müller, R.H. (1996). Nanopartículas de lípidos sólidos - Sistema coloidal alternativo para veiculação e libertação controlada de fármacos. Congresso Nacional de Farmacêuticos ‘96/3º Congresso Mundial de Farmacêuticos de Língua Portuguesa (Lisbon, Portugal). Abstract book, pp. 109. 11. Almeida, A.J., Runge, S., Müller, R.H. (1996). Estudos de encapsulação de fármacos de natureza peptídica em nanopartículas de lípidos sólidos. Congresso Nacional de Farmacêuticos ‘96/3º Congresso Mundial de Farmacêuticos de Língua Portuguesa (Lisbon, Portugal). Abstract book, pp. 112. 12. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1996). Mini-grânulos gastro-resistentes contendo AINEs produzidos por precipitação: I. Parâmetros de produção. Congresso Nacional de Farmacêuticos ‘96/3º Congresso Mundial de Farmacêuticos de Língua Portuguesa (Lisbon, Portugal). Abstract book, pp. 110. 13. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1996). Mini-grânulos gastro-resistentes contendo AINEs produzidos por precipitação: II. Caracterização. Congresso Nacional de Farmacêuticos ‘96/3º Congresso Mundial de Farmacêuticos de Língua Portuguesa (Lisbon, Portugal). Abstract book, pp. 113. 14. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1997). In vitro release studies on enteric granular formulations based on HP50. II Congreso Hispano-Luso de Liberación Controlada de Medicamento (Tenerife, Spain). Abstract book, pp. 119-120. 15. Cerdeira, A.M., Gouveia, L.F., Goucha, P., Almeida, A.J. (1997). Optimisation of the formulation of enteric beads prepared by the HP50 precipitation method. II Congreso 9 Hispano-Luso de Liberación Controlada de Medicamentos (Tenerife, Spain). Abstract book, pp. 117-118. 16. Gouveia, L.F., Cerdeira, A.M., Almeida, A.J., Morais, J.A.G. (1997). Determination of dissolution profiles of etodolac in enteric granules by flow injection analysis. II Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Tenerife, Spain). Abstract book, pp. 41-42. 17. Almeida, A.J., Runge, S., Müller, R.H. (1997). Investigations on solid lipid nanoparticles (SLN) as colloidal carriers for protein antigens. Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 24: 837-838 (Stockhom, Sweden). 18. Morais, J.A.G., Almeida, A.J., Cabral Marques, H.M., Cabrita, J.J., Lobato, M.R., Maya, M.T., Pinto, J.F., Rodrigues, L. (1998). Unidade de ciências e tecnologia farmacêuticas. I Encontro: Opportunities for Pharmaceutical R&D in Portugal (Oeiras, Portugal). Abstract book, P-III-17. 19. Videira, M., Azevedo, A.F., Almeida, A.J. (1998). Entrapment of a high molecular weight protein into solid lipid nanoparticles. Proceed. 2nd World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology (Paris, France), pp. 629-630. 20. Gouveia, L.F., Videira, M., Almeida, A.J. (1998). Determination of diltiazem in solid lipid nanoparticles by flow injection analysis with liquid-liquid extraction. Proceed. 2nd World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology (Paris, France), pp. 631-632. 21. Videira, M., Almeida, A.J. (1998). Can solid lipid nanoparticles be produced by a simple emulsion/solidification technique? III Congresso Hispano-Luso de Libertação Controlada de Medicamentos (Lisbon, Portugal). Abstract book, pp. 125-126. 22. Almeida, A.J., Almeida, A.P.G., Carrondo, M.J.T., Collares-Pereira, M., Cunha, A., Cruz, P., Clemente, A., Gonçalves, L.M.D., Moniz-Pereira, J., Novo, C., Oliveira, J., Santos-Gomes, G. (1999). Development of diagnostic kits and vaccines for infectious diseases. European Conference on Perspectives on Infectious Disease Research (Dresden, Germany). 23. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1999). Crystal size and habit influence on drug release from enteric beads produced by droplet extrusion/precipitation. Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 26: 1022-1023 (Boston, USA). 24. Videira, M., Botelho, F, Pedroso de Lima, J.J. Santos, A.C., Almeida, A.J. (1999). Lymphatic uptake of radiolabelled solid lipid nanoparticles administered by the pulmonary route. European Association of Nuclear Medicine Congress (Barcelona, Spain). Eur. J. Nucl. Med., 26: 1168. 25. Azevedo, A.F., Almeida, A.J. (1999). Encapsulation efficiency of proteins in PLGA microspheres by spray-drying: a factorial design approach. I Mediterranean CRS Conference: Drug Delivery in the Third Millennium (Pisa, Italy). Acta Tecnologiae et Legis Medicamenti, 10: 111. 26. Almeida, P.J., Almeida A.J. (1999). Alginate beads as an oral delivery system for βadrenergic blocking drugs: a preliminary study. I Mediterranean CRS Conference: Drug Delivery in the Third Millennium (Pisa, Italy). Acta Tecnologiae et Legis Medicamenti, 10: 112. 10 27. Videira, M., Almeida, A.J., Botelho, F., Santos, A.C., Gomes, C.M., Pedroso de Lima, J.J. (1999). SLN as a colloidal carrier system to the lung lymphatics. I Mediterranean CRS Conference: Drug Delivery in the Third Millennium (Pisa, Italy). Acta Tecnologiae et Legis Medicamenti, 10: 151. 28. Videira, M., Almeida, A.J., Müller, R.H. (2000). Incorporation of paclitaxel in SLN®: assessment of drug-lipid interaction. Proceed. 3rd Word Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology (Berlin, Germany), pp. 453-454. 29. Gouveia, L.F., Videira, M., Almeida, A.J., Morais, J.A. (2000). Determination of dissolution profiles of diltiazem tablets and capsules by FIA. 8th International Conference on Flow Analysis (Warsaw, Poland). Abstract book, pp. 287. 30. Almeida, A.J., Cerdeira A.M., Goucha, P. (2000) Development of a controlled release particulate formulation for oral delivery of NSAIDs. IV Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Vitoria, Spain). Abstract book, pp. 45-46. 31. Almeida, P.J., Almeida A.J. (2001). Mini-grânulos de alginato de cálcio produzidos por gelificação ionotrópica para a veículação de pindolol. II Jornadas da Sociedade Portuguesa de Ciências Farmacêuticas (Lisbon, Portugal). Abstract book, pp. A52. 32. Videira, M.A., Gano, L., Santos, C., Neves, M.A., Almeida, A.J. (2001). Estudos de biodistribuição de nanopartículas de lipídicas administradas por via endotraqueal. II Jornadas da Sociedade Portuguesa de Ciências Farmacêuticas (Lisbon, Portugal). Abstract book, pp. A68. 33. Salgado, A.C., Rosa, M.L.D., Almeida, A.J. (2001). Estabilidade do captopril em formulação magistral líquida para uso pediátrico obtida a partir de comprimidos. IV Congresso Nacional de Farmacêuticos Hospitalares (Oporto, Portugal). Livro de resumos, pp. 41. 34. Azevedo, A.F., Galhardas, J., Cunha, A., Cruz, P., Gonçalves, L.M.D., Almeida, A.J. (2002). Induction of protective immunity against strangles by immunisation with a S. equi enzymatic extract encapsulated in PLGA microparticles. Proceed. 4th Word Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Technology (Florence, Italy), pp. 881-882. 35. Rodrigues, M.A, Peiriço, N.M., Matos, H.A, Almeida, A.J., Gomes de Azevedo, E. (2002). Microparticles for controlled drug delivery systems using supercritical carbon dioxide. Proceed. 19th European Seminar on Applied Thermodynamics. (Santorini, Greece). 36. Ferreira Almeida, P., Almeida, A.J. (2002). Cross-linked calcium alginate particles for controlled release of drugs. World Congress of Pharmacy and Pharmaceutical Sciences 2002 (Nice, France). Abstract book, pp. 28. 37. Azevedo, A.F., Cunha, A., Almeida, A.J. (2002). Biodegradable microspheres containing S. equi antigens for immunisation against strangles. V Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Seville, Spain). Abstract book, pp. 79-80. 38. Lavrador, C. Alfaro Cardoso, L., Almeida, A.J. (2002) In vitro evaluation of a veterinary sustained-release injectable formulation of a β-agonist drug. V Congreso Hispano-Luso de Liberación Controlada de Medicamentos (Seville, Spain). Abstract book, pp. 123-124. 39. Videira, M., Pires, M.J., Soares, M., Almeida, A.J. (2003). Determination of particle concentration in solid lipid particle dispersions. 1º Congresso da Sociedade Portuguesa de Ciências Farmacêuticas (Lisbon, Portugal). Rev. Port. Farm., 52 (Supl.1): 122. 11 40. Ferreira Almeida, P.J., Almeida, A.J. (2003). Efeito da redução do tamanho de partícula na velocidade de libertação do pindolol em formulações de alginato de cálcio/gelatina. 1º Congresso da Sociedade Portuguesa de Ciências Farmacêuticas (Lisbon, Portugal). Rev. Port. Farm., 52 (Supl.1): 123. 41. Videira, M., Santos, A.C., Almeida, A.J. (2003). Stability studies on 99mTc-HMPAO-SLN: a particulate complex for lung and lymphatic imaging. Proceed. Intern. Symp. Control. Rel. Bioact. Mater., 30: (Glasgow, UK), pp. 337-338. 42. Salgado, A.C., Ribeiro, C., Rosa, M.L.D., Duarte, M.A., Almeida, A.J. (2003). Estabilidade de uma suspensão oral de espironolactona preparada a partir de comprimidos. IX Simposium Nacional de Farmacêuticos Hospitalares (Oporto, Portugal). Abstract book, pp. 31. 43. Toscano, C., Videira, M., Farinha, A, Rodrigues, L.M., Almeida, A.J. (2003). Encapsulação de substâncias com actividade fotoprotectora em nanopartículas lipídicas. Congresso Nacional dos Farmacêuticos 2003 (Lisbon, Portugal). Abstract book, pp. 205. 44. Pires, M. J., Videira, M., Almeida, A.J., Gouveia, L.F. (2003) ExcelTM add-in para análise de granulométrica de pós por tamisação e sedimentação. Congresso Nacional dos Farmacêuticos 2003, (Lisbon, Portugal). Abstract book, pp. 213. 45. Rodrigues, M.A, Li, J., Almeida, A.J., Matos, H.A., Gomes de Azevedo, E. (2004) Efficiencies of water removal from water/ethanol mixtures using supercritical carbon dioxide. V Encontro Brasileiro de Fluidos Supercríticos, (Florianópolis, Brazil). Abstract book, PP-063. 46. Toscano, C., Videira, M., Farinha, A, Rodrigues, L.M., Almeida, A.J. (2004). In vitro characterisation of solid lipid nanoparticles as a carrier system for photoprotective agents. European Conference on Drug Delivery and Pharmaceutical Technology (Seville, Spain). Abstract book, pp. 82. 47. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2004). Determinação analítica do teor em elementos minerais essenciais e tóxicos nas bebidas alcoólicas mais consumidas em Portugal. International Conference on Food Protection (Lisbon, Portugal). Abstract book. 48. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2004). Perfil qualitativo e quantitativo dos minerais em refrigerantes e néctares. International Conference on Food Protection (Lisbon, Portugal). Abstract book. 49. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2004). Leite materno determinação de elementos minerais essenciais e tóxicos. 7º Congresso Português de Pediatria, (Lisbon, Portugal). Acta Pediátrica Portuguesa (publication in CD-ROM). 50. Ferreira Almeida, P.J., Almeida, A.J. (2004). Pindolol-containing alginate-gelatine microparticles as an oral controlled release system. 8th European Congress of Pharmaceutical Sciences (EUFEPS 2004), Brussels, Belgium. Eur. J. Pharm. Sci., 23 (Suppl. 1): S51. 51. Florindo, H.F., Almeida, A.J. (2004). Protein release from alginate microparticles: disintegration studies and release mechanisms, 8th European Congress of Pharmaceutical Sciences (EUFEPS 2004), Brussels, Belgium. Eur. J. Pharm. Sci., 23 (Suppl. 1): S64. 52. Toscano, C., Farinha, A, Pinto, P.C., Magro, J.M., Rei, F., Almeida, A.J., Rodrigues, (2004). In vivo evaluation of photoprotective activity of a solid lipid nanoparticle formulation. 8th 12 European Congress of Pharmaceutical Sciences (EUFEPS 2004), Brussels, Belgium. Eur. J. Pharm. Sci., 23 (Suppl. 1): S47-S48. 53. Conceição, M., Nascimento, C., Almeida, A.J., Tavares de Almeida, I., Leandro, P. (2004). Preserving human phenylalanine hydroxylase activity: mechanism of polyol-induced in vitro stabilisation. 3rd Portuguese-Spanish Biophysics Congress (Lisbon, Portugal). Abstract book, pp.65. 54. Salgado, A.C., Rosa, M.L.D., Duarte, M.A., Almeida, A.J. (2004). A importância da qualidade microbiológica na avaliação da estabilidade de uma suspensão oral de espironolactona para uso pediátrico. V Congresso da APFH (Lisbon, Portugal). Abstract book, pp. 11. 55. Salgado, A.C., Rosa, M.L.D., Duarte, M.A., Almeida, A.J. (2004). Estabilidade de uma suspensão oral de ranitidina preparada a partir de comprimidos. V Congresso da APFH, (Lisbon, Portugal). Abstract book, pp. 11. 56. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2005). Leite materno – Contributo do pescado para a presença de mercúrio. 7º Encontro de Química dos Alimentos (Viseu, Portugal). Abstract book, pp. 168. 57. Toscano, C., Farinha, A., Pinto, P.C., Magro, J.M., Rei, F., Almeida, A.J., Rodrigues, (2005). Biological effects of lipid nanoparticles on skin photobiology. II Congress of the Portuguese Society of Pharmaceutical Sciences/VI Congress of the Portuguese-Spanish Chapter of the Controlled Release Society (Coimbra, Portugal). Rev. Port. Far. 52 (Supl. 2): 184-185. 58. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2005). Selénio e leite materno. II Congresso do Departamento das Ciências e Tecnologias Laboratoriais e Intervenção Comunitária, Instituto Politécnico de Lisboa (Lisbon, Portugal). Abstract book, pp. 96. 59. Rodrigues, L.M.,Toscano, C., Farinha, A., Pinto, P.C., Magro, J.M., Rei, F., Almeida, A.J., (2005). Biological effects of lipid nanoparticles on in vivo skin biology. 4th International Academy of Cosmetic Dermatology World Congress (Paris, France). Les Nouvelles Dermatologiques, 24 (Sup. 7): 74. 60. Florindo H.F., Pandit, S., Gonçalves, L.M.D., Somavarapu, S., Almeida, A.J., Alpar, H.O. (2005). S. equi enzymatic extract adsorbed to polycaprolactone microspheres: production, characterization and immune response in mice. 15th International Symposium on Microencapsulation (Parma, Italy). Abstract book, pp. 339-340. 61. Albuquerque, M., Almeida, A.J. (2005). Preparação e avaliação de uma forma magistral líquida oral de pirazinamida. Congresso do Hospital de D. Estefânia 2005: Inovar e Humanizar (Lisboa, Portugal). Abstract book, pp. 49. 62. Azevedo A.F., Galhardas J., Cunha A., Cruz P., Gonçalves L.M.D., Almeida A.J. (2005). Immobilisation of Streptococcus equi antigens in biodegradable microspheres induces protection against experimental infection in mice. MicroBiotec’05 (Póvoa de Varzim, Portugal). Abstract book, pp. 375. 63. Florindo H.F., Pandit S., Gonçalves L.M.D., Almeida A.J., Alpar O. (2005). Adsorption studies of Streptococcus equi antigens onto biodegradable micro/nanoparticles. MicroBiotec’05 (Póvoa de Varzim, Portugal). Abstract book, pp. 419. 13 64. Florindo, H.F., Gonçalves, L.M.D., Alpar, H.O., Almeida, A.J. (2006). Adsorption kinetics of Streptococcus equi enzymatic extract onto surface modified PLA nanospheres. Proc. Intern. Symp. Control. Rel. Bioact. Mater., 33: (Viena, Austria). P-200 (in CD-ROM). 65. Ferreira Almeida, P., Moita, J., Almeida, A.J. (2006). Gastroresistent crosslinked alginategelatine particles. Proc. Intern. Symp. Control. Rel. Bioact. Mater., 33: (Viena, Austria). P844 (in CD-ROM). 66. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). Feasibility studies of lipid-based carriers for dermal applications. XIV International Workshop on Bioencapsulation (Lausanne, Suwitzerland). 67. Barros, C.T., Almeida, A.J., Contente, M. (2006). Extemporaneous formulation of oral paediatric medicines in Portuguese hospitals. 35th European Symposium on Clinical Pharmacy. (Viena, Austria). 68. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). Controlled release of cyclosporine A from triacylglycerol-based lipid nanoparticles VII Congress Portuguese-Spanish Congress on Controlled Drug Delivery (Pamplona, Spain). Abstract book, pp. 141-142. 69. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). Solid lipid microparticles (SLM) versus nanoparticles (SLN): effects on reduction of volatility of essential oils. 2006 AAPS Annual Meeting and Exposition (San Antonio, USA). AAPS Journal, 8 (S2): Abstract M1146. 70. Silva, A., Albuquerque, M., Salgado, A, Almeida, A.J., Duarte, M.A. (2006). Estudo da actividade bactericida da pirazinamida em estirpes hospitalares multiresistentes numa formulação magistral. 11º Simpósio Nacional da APFH, (Estoril, Portugal). Abstract book, pp. 37. 71. Mendes, L., Rodrigues, C., Almeida, A.J., Castro, M., Soveral, G. (2006). Mercury and human milk. XX Encontro Nacional da SPQ (Campus da Caparica, Portugal). Abstract book, pp. 258. 72. Nascimento, C., Ramos, L., Almeida, A.J., Tavares de Almeida, I., Leandro, P. (2006). Effect of addictives on the biological function of recombinant human phenylalanine hydroxylase: solution and lyophilisation assays. XV Nacional Congress of Biochemistry (Aveiro, Portugal). Abstract book, vol. 2, pp. 91. 4.4. PAPERS PUBLISHED IN CONFERENCE PROCEEDINGS 1. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1992). The efficient encapsulation of biologically intact high molecular weight proteins into submicron polylactide microspheres. Proceed. 6th International Conference on Pharmarmaceutical Technology, Vol. I, pp. 35-43. 2. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1992). Protein adsorbed polylactide microspheres as mucosal immunoadjuvants. Proceed. 6th International Conference on Pharmaceutical Technology, Vol. I, pp. 44-51. 3. Cerdeira, A.M., Brandão, A., Goucha, P., Cabral Marques, H.M., Almeida, A.J. (1998). Preparation and characterisation of beads containing a nabumetone: dimethyl-β-cyclodextrin complex. Proceed. 17th Pharmaceutical Technology Conference, Vol. I, pp. 42-51. 14 4. Rodrigues, M.A., Peiriço, N.M., Matos, H.A., Almeida, A.J., Gomes de Azevedo, E. (2002). Microparticles for controlled drug delivery systems using supercritical carbon dioxide. Proceed. 19th European Seminar on Applied Thermodynamics, pp.144-147. 5. Rodrigues, M.A., Li, J., Almeida, A.J., Matos, H.A., Gomes de Azevedo, E. (2004) Efficiencies of water removal from water/ethanol mixtures using supercritical carbon dioxide. V Encontro Brasileiro de Fluidos Supercríticos, (in CD-ROM). 6. Almeida, A.J., Toscano, C., Videira, M. (2004). Therapeutic applications of lipid nanoparticles administered by alternative routes. In: Pedraz, J.L., Orive, G., Poncelet, D. (eds), XII International Workshop on Bioencapsulation, pp. 69-72. 7. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). SLN and NLC as viscoelastic enhancers for topical drug delivery. Proc. XIV International Workshop on Bioencapsulation & COST 865 meeting (Lausanne, Switzerland) O6-5. 8. Souto, E.B., Almeida, A.J., Müller, R.H. (2006). Feasibility studies of lipid-based carriers for dermal applications. Proc. XIV International Workshop on Bioencapsulation & COST 865 meeting (Lausanne, Switzerland). P-27 4.5. PAPERS IN JOURNALS WITH REFEREES 1. Almeida, A.J., Alpar, H.O., Brown, M.R.W. (1993). Immune response to nasal delivery of antigenically intact tetanus toxoid associated with poly(L-lactic acid) microspheres in rats, rabbits and guinea-pigs. Journal of Pharmacy and Pharmacology, 45: 198-203. 2. Alpar, H.O., Almeida, A.J., Brown, M.R.W. (1994). Microsphere absorption by the nasal mucosa of the rat. Journal of Drug Targeting, 2: 147-149. 3. Alpar, H.O., Almeida, A.J. (1994). Identification of some physico-chemical characteristics of microspheres, which influence the induction of the immune response following mucosal delivery. European Journal of Pharmaceutics and Biopharmaceutics, 40: 198-202. 4. Almeida, A.J. (1994). Vaccine delivery systems. Revista Portuguesa de Farmácia, 44: 3-13. 5. Poyner, E.A., Alpar, H.O., Almeida, A.J., Gamble, M.D., Brown, M.R.W. (1995). A comparative study on the pulmonary delivery of tobramycin encapsulated into liposomes and PLA microspheres following intravenous and endotracheal delivery. Journal of Controlled Release, 31: 41-48. 6. Almeida, A.J., Alpar, H.O. (1996). Nasal delivery of vaccines. Journal of Drug Targeting, 3: 455-467. 7. Almeida, A.J., Runge, S., Müller, R.H. (1997). Peptide-loaded solid lipid nanoparticles (SLN): influence of production parameters. International Journal of Pharmaceutics, 149: 255-265. 8. Cerdeira, A.M., Goucha, P., Almeida, A.J. (1998). Hydroxypropyl methylcellulose phthalate beads containing a model non-steroid anti-inflammatory drug. International Journal of Pharmaceutics, 164: 147-154. 9. Paulo, M.G., Cabral Marques, H.M., Morais, J.A.G., Almeida, A.J. (1999). An isocratic LC method for the simultaneous determination of vitamins A, C, E and β-carotene. Journal of Pharmaceutical and Biomedical Analysis, 21: 399-406. 15 10. Cerdeira, A.M., Gouveia, L.F., Goucha, P., Almeida, A.J. (2000). Drug particle size influence on enteric beads produced by a droplet extrusion/precipitation method. Journal of Microencapsulation, 17: 733-741. 11. Lavrador, C., Almeida, A.J., Cardoso, L.A. (2002). Avaliação in vitro de um injectável subcutâneo para libertação prolongada de um fármaco β-agonista. Revista Portuguesa de Ciências Veterinárias, 97: 29-34. 12. Videira, M., Botelho, M.F., Santos, A.C., Gomes, C.M., Pedroso de Lima, J.J., Almeida, A.J. (2002). Pulmonary delivery of SLN as a colloidal drug carrier system to the lung lymphatics. Journal of Drug Targeting, 10: 607-613. 13. Rodrigues, M., Peiriço, N., Matos, H., Gomes de Azevedo, E., Lobato, M.R., Almeida, A.J. (2004). Microcomposites theophylline/hydrogenated palm oil from a PGSS process for controlled drug delivery systems. Journal of Supercritical Fluids, 29: 175-184. 14. Ferreira Almeida, P.J., Almeida A.J. (2004). Cross-linked alginate-gelatine beads: a new matrix for controlled release of pindolol. Journal of Controlled Release, 97: 431-439. 15. Salgado, A.C., Rosa, M.L., Duarte, M.A., Almeida, A.J. (2005). Stability of spironolactone in an extemporaneously prepared aqueous suspension: importance of microbiological quality of compounded paediatric formulations. European Journal of Hospital Pharmacy 11: 68-73. 16. Rodrigues, M., Li, J., Almeida, A.J., Matos, H.A., Gomes de Azevedo, E. (2006). Efficiencies of water removal from water/ethanol mixtures using supercritical carbon dioxide. Brazilian Journal of Chemical Engineering, 23: 205-212. 17. Azevedo, A.F., Galhardas, J., Cunha, A., Cruz, P., Gonçalves, L.M.D., Almeida, A.J. (2006). Microencapsulation of Streptococcus equi antigens in biodegradable microspheres and preliminary immunisation studies. European Journal of Pharmaceutics and Biopharmaceutics, 64: 131-137. 18. Videira, M.A., Gano, L., Santos, C., Neves, M., Almeida, A.J. (2006). Lymphatic uptake of lipid nanoparticles following endotracheal administration to rats. Journal of Microencapsulation, 23: 855-862. 19. Rodrigues, M., Jun, L., Matos, H., Almeida, A.J., Gomes de Azevedo, E. (2007). Control of the morphology of microparticles produced by supercritical CO2 from water/ethanol solutions. (submitted). 20. Almeida, A.J., Souto, E. (2007). Solid lipid nanoparticles as drug delivery systems for peptides and proteins. Advanced Drug Delivery Reviews (submitted) 21. Souto, E., Almeida, A.J., Müller, R.H. (2007). Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: structure, protection and skin effects. Advanced Drug Delivery Reviews (submitted). 4.6. 1. BOOK CHAPTERS Almeida, A.J., Alpar, H.O. (1997). Mucosal immunisation with antigen-containing microparticles. In: Gander, B., Merkle, H.P. and Corradin, G. (eds.), Antigen Delivery Systems: Immunological and Technological Issues. Harwood Academic Publishers, Amsterdam. pp. 207-226. 16 2. Cruz, M.E.M., Almeida, A.J., Corvo, M.L. (2003). Sistemas de libertação controlada de fármacos. In: Lima, N. and Mota, M. (eds.), Biotecnologia: Fundamentos e Aplicações. Lidel - Edições Técnicas, Lisboa. pp. 359-376. 4.7. PATENTS 1. Goucha, P., Cerdeira, A.M., Almeida, A.J. (2000). Process for formulating granular matrices by precipitation of polymers in an acidic medium. European Patent EP 1018335. 2. Goucha, P., Cerdeira, A.M., Almeida, A.J. (2000). Apparatus for the preparation of spherical polymer particles. European Patent EP 1018364. 3. Ferreira Almeida, P., Almeida A.J. (2004). Partículas de um polissacárido e uma poliamina interligados por um agente de cross-linking, contendo uma substância activa para libertação lenta. Portuguese Patent nº 102728. 4.8. OTHER PUBLICATIONS 1. Almeida, A.J. (1995). Microemulsões como sistemas terapêuticos para a administração oral de péptidos. Notícias de Farmácia Hospitalar. 11: 5-6. 2. Almeida, A.J., Morais, J.A.G. (1999). Guia dos principais medicamentos. In: Tratamentos Médicos e Alternativos. Selecções do Reader’s Digest, Lisboa, pp. 444-466 (translation). 3. Salgado, A.C., Rosa, M.L.D., Almeida, A.J. (2002). Estabilidade do captopril em formulação magistral líquida para uso pediátrico obtida a partir de comprimidos. Boletim da APFH, 52: 2-4. 4. Salgado, A.C., Rosa, M.L.D., Almeida, A.J. (2003). Estabilidade do cloridrato de ranitidina em formulação magistral líquida para uso pediátrico obtida a partir de comprimidos. Boletim da APFH, 74, 7-10. 5. Almeida, A.J. (2005). Formulação magistral para necessidades hospitalares específicas: pediatria. Mundo Farmacêutico, 15: 52-54. 6. Almeida, A.J. (2006). Estratégias de utilização de microesferas e nanopartículas em vacinas. Mundo Farmacêutico, 25: 26-28. 5. GRANTS 1982/86 - Fundação Calouste Gulbenkian – grant for pregraduation studies. 1986/87 - Atral-Cipan, S.A. – pharmaceutical industry grant for research. 1987/88 - Instituto Nacional de Investigação Agrária / Instituto Gulbenkian de Ciência (Fundo Social Europeu) – grant for research. 1990/91 - INVOTAN – NATO grant for PhD studies in the UK. 1991/93 - JNICT (Programa Ciência) - grant for PhD studies in the UK. 1995 - Deutscher Akademischer Austauschdienst (DAAD) – Postdoctoral grant for research in Germany. 1996 - INVOTAN – NATO grant for research in Germany. 17 6. AWARDS 1989 - Military award for services at the military laboratory during national service. 2001 - The APFH-Farmoz Award for the best poster presentation at the IV Congresso Nacional de Farmacêuticos Hospitalares (Oporto, Portugal):“Salgado, A.C., Rosa, M.L.D. e Almeida, A.J., Estabilidade do captopril em formulação magistral líquida para uso pediátrico obtida a partir de comprimidos”. 2002 - Scientific Merit Award, to the work “Salgado, A.C., Rosa, M.L.D. e Almeida, A.J., Estabilidade do captopril em formulação magistral líquida para uso pediátrico obtida a partir de comprimidos”, on the 125th aniversary to the Paediatric Hospital (Hospital de D. Estefânia). 2005 - Best Presentation Award to the presentation “Albuquerque, M., Almeida, A.J., Preparação e avaliação de uma forma magistral líquida oral de pirazinamida”, Congresso do Hospital de D. Estefânia 2005. 2006 - Best Oral Presentation Award a the VII Congress Portuguese-Spanish Congress on Controlled Drug Delivery (Pamplona, Spain, 22-24 October) to the presentation Florindo, H.F., Gonçalves, L.M.D., Alpar, H.O., Almeida, A.J. Adsorption of Streptococcus equi enzymatic extract on the surface modified PCL nanospheres. Best Poster Award at 11º Simpósio Nacional da Associação Portuguesa de Farmacêuticos Hospitalares (Estoril, 8-11 November) to the presentaion “Silva, A., Albuquerque, M., Salgado, A, Almeida, A.J., Duarte, M.A. Estudo da actividade bactericida da pirazinamida em estirpes hospitalares multiresistentes numa formulação magistral. 7. SCIENTIFIC SOCIETIES ♦ ♦ ♦ ♦ ♦ ♦ Portuguese Pharmaceutical Society (Ordem dos Farmacêuticos; since 1987). Portuguese Biochemical Society (Sociedade Portuguesa de Bioquímica; since 1987). Portuguese Biotechnological Society (Sociedade Portuguesa de Biotecnologia; since 1987). Controlled Release Society (CRS; since 1993). Founder of the Spanish-Portuguese Local Chapter (1995). International Association for Pharmaceutical Technology (APV; since 1995). Portuguese Society for Pharmaceutical Sciences (Sociedade Portuguesa de Ciências Farmacêuticas; founder in 1998). 18
Download