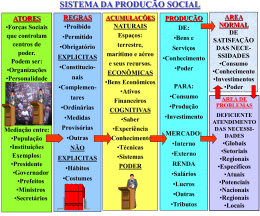
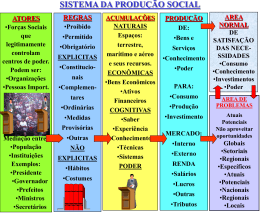
MSc. Jorge Mendonça Líder e coordenador projeto FACT BRASIL Far-Manguinhos / FIOCRUZ 17de Abril de 2008 Um Projeto Global Rede FACT Modelo colaborativo da DNDi ilustrado pelo Projeto FACT Uni Bordeaux, France: desenvolvimento farmacêutico de AS/AQ CNRFP, Burkina Faso: ensaio clínico de AS/AQ Uni Sains, Malaysia: devt of metodologia analítica, PK humanos & animal Far Manguinhos, Brazil: desenvolvimento farmacêutico de AS/MQ, toxicologia AS/AQ: artesunato/am odiaquina AS/MQ: artesunato/mefloquina PK/PD: farm acocinética/farm acodinâmica DNDi, MSF, TDR, Far-Manguinhos, EC’s INCODEV: financiam ento DNDi/TDR: coordenação científica e gestão de projeto Uni Oxford, UK: PK/PD, estudos in vitro and moleculares Uni Mahidol, Thailand: ensaio clínico de AS/MQ -- - - - - Inform ação compartilhada ----------- Inform ação/dados/m étodos com partilhados Um produto Global ASMQ O comprimido azul dos trópicos • América do Sul; • Ásia; • Possivelmente África. FARMANGUINHOS MISSÃO:Contribuir para a promoção da saúde pública, por meio da produção de medicamentos, do desenvolvimento tecnológico e difusão de conhecimentos. VISÃO: Ser um centro de referência da pesquisa, do desenvolvimento e da produção farmacêutica brasileira. FOCO: Priorizar o acesso da população brasileira aos programas governamentais de saúde. ÁREAS ENVOLVIDAS NO PROJETO FACT AAR AAR NVQ NVQ LTF GQ GQ LDVA COMERCIAL COMERCIAL PCP PCP CQ CQ PRODUÇÃO PRODUÇÃO Controle Controle da da Qualidade Qualidade Ministério Ministérioda daSaúde Saúde PMA PMA ENGENHARIA ENGENHARIA Estrutural Organizacional FACT - Farmanguinhos Tecnologia Farmacêutica LTF Alessandra Viçosa Qualidade LDVA /NVQ Érico Daemon Coordenador local – Jorge Mendonça Consultores do projeto (CATALENT, Dra Isabela Ribeiro e Dra. Luciana Barros) Jorge Mendonça Far-Manguinhos / FIOCRUZ 17de Abril de 2008 ASMQ The development of a co-formulation FROM THE BLUEPRINT TO THE BLUE PILL BLUEPRINT ? BLUE PILL 9 THE BLUEPRINT OF THE BLUE PILL BLUEPRINT • Quality components (AS, MQ, Excipients) • Smallest possible size (Minimum excipients) • Good aspect (Coating) • Paediatric strengths • Simple (1 or 2 tables for 3 days) • Stable (Process and Tropical conditions) • Adequate biopharmaceutical properties. 10 APIs Characteristics ? MEFLOQUINE HYDROCHLORIDE ARTESUNATE X Stability: Not Critical issue Stability: Critical issue 11 Mefloquine Hydrochloride Manufacturer: Abbott GmBH & Co. KG - Germany/ Sifavitor – Italy (New supplier) E-DMF available, Comply with EP Exists in known different polymorphs (A,B,C,D and E) Other Preformulation tests: Bulk and Tapped density Hausner ratio: Fair Carr index: Fair Repose angle: Poor Water content: Máx 3 % Particle size distribution Thermal analysis X – ray Problem: Poor Flow ! 12 Artesunate Manufacturer: Knoll AG - Switzerland E-DMF available, FDA inspected Manufacturing Site, Comply with IP. Artesunate does not show polymorphism. Particle size profile (Knoll) Batches 2.05 4.05 d50 = 77µ 77µm d50 = 132µ 132µm Different Particle size profile !!! 13 Formulation design Smallest excipient quantities Smallest tablet size Stable formulation: APIs x Excipients compatibility Simple composition Artesunate - API Mefloquine Hydrochloride - API APIs - About 70 % of the formulation Microcrystalline cellulose - Diluent Sodium croscarmellose - Disintegrant Magnesium stearate – Lubricant Opadry white - Coating Blue Lake FDC 2 - Coating Excipients - About 30 % of the formulation Coating function: Taste masking , light protection and good aspect 14 High dosage X Low dosage Same formulation and different average mass Average mass/4 = Low Dosage Artesunate + Mefloquine (25 +55) mg Fast disintegration time High Dosage Artesunate + Mefloquine (100 +220) mg No problems to child administration in the spoon !! 15 And about the quality of excipients? Microcrystalline cellulose – Comply with EP Sodium croscarmellose - Comply with EP Magnesium stearate – Comply with EP - Animal origin Very important to good processability in production area. Opadry white Blue Lake FDC 2 Pharmaceutical grade The nice aspect of the BLUE tablets required this kind of materials. 16 Process Choice/ Optimizing Stability Mefloquine Dry Granulation Mixing To improve MQ flow Addition of Artesunate – Dry process Tabletting Coating Packaging Aluminium foil High Humidity Protection Clinical Trial packaging 17 Dosing Schedule 18 Packaging (Simplicity and Patient Compliance) 6 to 11 months 1 – 5 years 6 – 11 years 12 years or older 19 DR profiles of tablets MQ release AS release 20 Stability / Liability of the product : stress testing •Stability-indicating assay and related substances method •Degradation peaks from artesunate • <1 % degradation to light and humidity •~3% of unknown impurities in oxidative conditions •Strong degradation with heat •Excipients not found to increase degradation Table 11. Qualitative comparision of chromatograms in solid state stress conditions Sample/Condition Control Sample Heat (Chamber) Humidity Light (non degraded) Artesunate -5 0 45 0 5 10 15 15 25 30 35 40 5 Name RetentionTime Area 25 30 35 40 45 -5 0 Minutes 5 10 15 20 5 10 10 15 Name RetentionTime Area 25 30 35 40 45 5 10 15 25 30 35 40 -5 0 45 Minutes 5 15 5 10 25 30 35 40 15 20 -5 0 45 5 35 40 -5 0 45 35 40 45 5 10 15 10 15 UNK 1 20 25 30 35 40 45 mAU 5 0 25 30 35 40 45 1:211nm ,8nm FACT comprimidospulvluz1 Name RetentionTime Area -5 0 20 UNK 2 DHA 1 M inutes 25 30 35 40 5 10 15 20 45 Minutes 15 10 Name RetentionTime Area 5 DetectorA(211nm) FACT Excipienteluz1 Name RetentionTime Area 5 40 25 Minutes 20 25 30 35 40 -5 0 45 -5 0 5 10 15 20 30 35 40 45 -5 0 5 10 15 10 5 0 20 25 Minutes 30 35 40 5 10 15 20 25 30 35 40 45 Minutes 45 30 35 40 45 -5 0 15 DetectorA(211nm) FACT 5comprimidosumid1 Name RetentionTime Area 10 m AU Name RetentionTime Area 25 Minutes 15 1:211nm,8nm FACT 5comprimidosaquec1 12.42 23533 5 0 20 15 9.22 22695 10 15 10 mAU Name Retention Time Area 10 5 Minutes 15 DetectorA(211nm) FACT compPApulvcontrole1 5 0 -5 0 45 5 0 -5 0 1:211nm ,8nm FACT 5com primidosluz1 Name RetentionTime Area 5 10.83 27331 35 8.50 9078 30 mefloquina 18.65 225093583 0 -5 0 30 Mefloquina 17.81 155464440 25 m AU 5 30 DetectorA(211nm) FACT Excipienteumid1 Artesunato 4.58 1088733 20 12. 13 55948 15 mefloquina 18.57 196539129 mAU 10 10 Mefloquina 16.80 147357702 10 artes unato 4.86 993530 Drug Product (5 tablets) a Name RetentionTime Area 5 0 5 Minutes 15 25 0 0 -5 0 25 mAU mAU 10 15 mAU Name RetentionTime Area 5 20 Minutes DetectorA(211nm) FACT Excipienteaquec2 8.80 19669 mAU 10 15 DetectorA(211nm) FACT Excipienteumid1 Art esunat o 4. 37 683638 15 Name RetentionTime Area 5 10 Minutes Placebo 15 Minutes 15 12.48 38138 Name RetentionTime Area 9.22 17399 5 0 20 10 DetectorA(211nm ) FACT M PMQluz1 0 mefloquina 18.71 227951463 10 mAU 12.10 91852 10 5 45 DetectorA(211nm) FACT comprimidospulvumid1 Artesunato 4.58 1092846 0 20 Name RetentionTime Area 8.79 69158 mAU 5 40 Minutes 15 Mefloquina 16.83 144992502 0 -5 0 10 35 10 0 20 30 15 5 -5 0 1:211nm,8nm FACT comprimidospulvaquec1 Artesunato 4.37 663291 5 mefloquina 18.57 196539129 mAU 10 15 DetectorA(211nm) FACT compPApulvcontrole1 Name Retention Time Area artes unato 4.86 993530 15 25 Minutes DetectorA(211nm) FACT MPMQumid1 Minutes Drug Product (pulverized) artesunato 4.28 1213890 m AU -5 0 45 15 0 20 20 Artesunato 4.17 683606 -5 0 mAU 5 10 DetectorA(211nm) FACT MPMQaquec1 mAU 10 Mefloquina 18.32 220675326 mAU 10 5 Minutes 15 mefloquina 17.77 231923498 40 11.00 22602 35 8.59 6157 30 MQ Mefloquina 17. 86 158308532 25 0 DetectorA(211nm ) FACT rMPAScontroleluz2 Name RetentionTime Area Artes unat o 4.17 607707 20 DetectorA(211nm) FACT MPMQcontrole1 Name Retention Time Area 5 -5 0 13.37 32306 15 10 18.52 233393882 10 Minutes 15 15 DetectorA(211nm) FACT MPAScontroleumid1 Name RetentionTime Area 5 0 mAU 5 0 12.24 18956 -5 0 Mefloquine 5 mefloquina 16.88 207082379 0 10 mAU 5 15 DetectorA(211nm) FACT uMPASaquec1 Name RetentionTime Area 4.51 1074375 mAU 10 artesunato 4.65 1222921 15 DetectorA(211nm) FACT MPAScontrole1 Name RetentionTime Area 4.80 1070536 mAU 10 (Artesunato) 15 10 15 HPLC-3D detector 20 25 30 35 40 45 Minutes 5 10 15 20 25 30 35 40 45 Minutes a = non pulverized, intacts 21 Stability Studies – 36 Months 22 The Product: 1st Fixed-Dose ACT with 3-Year Shelf Life 23 Manufacturing History and Status Batches BatchesSize Size Batches 2x BatchesSize Size Batches BatchesSize Size BatchesSize Size Batches 2x100 100Kg Kg 2x 16 Kg 2x 2x 44xx16 2x 16 Kg 2x12 12Kg Kg 2x16 16Kg Kg 16Kg Kg 2006 2004 Stability Studies Acre/Pará Clinical Trials Drugs used in: - Thailand Studies - Acre/Pará Clinical Trials 2007 Jan/ 2008 Acre Clinical Trials New MQ Supplier Batches Validation Validation Batches Batches ri p A 08 0 l2 ! Market Batches 24 THE BLUE PILL Quality components (AS, MQ, Excipients) Smallest possible size (Minimum excipients) Good aspect (Coating) Paediatric strengths Simple (1 or 2 tables for 3 days) Stable (Process and Tropical conditions) Adequate biopharmaceutical properties. 25 Jorge Mendonça Far-Manguinhos / FIOCRUZ 17de Abril de 2008 Conceitos do projeto • Facilidade de Uso (1 ou 2 cps por dia); • Uso pediátrico; • Menor tamanho possível e bom aspecto; •Estável tanto em processo quanto em condições tropicais; • Mais barato que os medicamentos isolados. PROCESSOS E PESSOAS TIME FACT TIME ANALÍTICO Estudo clínico na Tailândia Estudo clínico no Acre Produção Produção Agradecimentos: • Farmanguinhos FIOCRUZ • DNDi • Dr. Jean-René Kiechel • Dra. Isabela Ribeiro • Eloan Pinheiro • Eduardo Costa • Dra. Núbia Boechat OBRIGADO PELA ATENÇÃO [email protected] (Coordenador) [email protected] (Tecnologia Farmacêutica) [email protected] (Qualidade) [email protected] (consultora)
Baixar