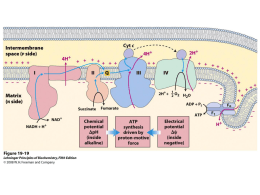
Voltammetric methods and electrodes Introduction Electroanalytical methods Interfacial methods Static methods I~0 Potentiometry (E) Const. electrode potential coulometry (Q = ∫01 idt Bulk methods Dinamic methods I#0 Conductometry (G = 1/R) Potentiometric titrations (volume) Controlled potential Voltammetry [ I = f (E) ] Conductometric Titrations (volume) Constant current Coulometric Titrations (Q = It) Amperometric titrations (volume) Electrogravimetry (wt) Electrogravimetry (wt) Five Important interrelated concepts to understand electrochemistry: (1) the electrode’s potential determines the analyte’s form at the electrode’s surface; (2) the concentration of analyte at the electrode’s surface may not be the same as its concentration in bulk solution; (3) in addition to an oxidation–reduction reaction, the analyte may participate in other reactions; (4) current is a measure of the rate of the analyte’s oxidation or reduction; and (5) we cannot simultaneously control current and potential. L. Faulkner, Understanding electrochemistry: some distinctive concepts,” J. Chem. Educ., 60, 262 (1983) ; P. T. Kissinger, A. W. Bott, Electrochemistry for the NonElectrochemist,” Current Separations, 20, 51 (2002) 1) The Electrode’s Potential Determines the Analyte’s Form Fig. Redox ladder diagram for Fe3+/Fe2+ and for Sn4+/ Sn2+ redox couples. The areas in blue show the potential range where the oxidized forms are the predominate species; the reduced forms are the predominate species in the areas shown in pink. Note that a more positive potential favors the oxidized forms. At a potential of +0.500 V (green arrow) Fe3+ reduces to Fe2+, but Sn4+ remains unchanged. 2) Interfacial Concentrations May Not Equal Bulk Concentrations Fig. Concentration of Fe3+ as a function of distance from the electrode’s surface at (a) E = +1.00 V and (b) E = +0.500 V. The electrode is shown in gray and the solution in blue. Nernst Equation E = Eo + 0.0592/n + log [ox]/[red] Fe3+ + e Fe2+ E = Eo + 0.0592/1 + log [Fe3+]/[Fe2+] 3) The Analyte May Participate in Other Reactions Fe3+ + OH- FeOH2+ 4) We Cannot Simultaneously Control Both Current and Potential 5) Controlling and Measuring Current and Potential Controlled Potential Methods (Voltammetry) Fig. Flow patterns and regions of interest near the work electrode in hydrodynamic voltammetry Controlled Potential Methods (Voltammetry) O + ne R E = Eo` + 0.0592/n + log CsO / CsR (1) (2) where E = potential applied to electrode (mV) Eo`= formal reduction potential of the couple vs Eref n = number of electrons in reaction (1) CsO = surface concentration of species O CsR = surface concentration of species R Table. Relationship of E to surface concentrations# E, mV CsO / CsR 236 10,000/1 177 1,000/1 118 100/1 59 10/1 0 1/1 -59 1/10 -118 1/100 -177 1/1,000 -236 1/10,000 For a reversible system, n = 1, Eo`= V The current at an electrode is related to the flux (rate of mass transfer) of material to the electrode (3) Considering x = and C = CO – CsO Where = Nernst diffusion layer (4) 4 (5) Where ilc is limiting cathodic current and CsO is zero Cyclic Voltammetry FeIII(CN)63- + e FeII(CN)64- FeII(CN)64- (1) FeIII(CN)63- (2) Nernst Equation for a reversible system E = Eo` + 0.0592/1 log [FeIII(CN)63-] / [FeII(CN)64-] (3) Eo` = E1/2 = (Epa + Epa)/ 2 (4) Ep = Epa – Epc = 0.059/ n (5) The peak current for a reversible system is described by RandlesSevcic Equation for the forward sweep for the first cicle: ip = 2.69 105 n2/3 A D1/2 1/2 C (6) Where: ip = peak current (A); n = number of electrons; A = electrode area (cm2); D = diffusion coefficient (cm2 / s); = scan rate (V /s) and C = concentration (mol / cm3) Cronoamperometria Figura (A) Representação esquemática da aplicação de potencial em voltametria de pulso diferencial. A corrente é amostrada em S1 e S2 e a diferença entre elas é registrada; (B) Voltamograma de pulso diferencial. SCHOLZ, F., ed (2005). Electroanalytical methods. New York: Springer. Figura (1) Forma de aplicação de potencial na voltametria de onda quadrada; (2) Voltamogramas de onda quadrada esquemáticos para um sistema reversível (A) e para um sistema totalmente irreversível (B). SOUZA, D.; MACHADO, S. A. S.; AVACA, L. A. Química Nova, Vol. 26, 81-89, 2003. LOVRIC, M.; KOMORSKY- LOVRIC, S.; MIRCESKI, V. Square Wave Voltammetry. ed. (2007), Berlin: Springer. Glassy carbon electrode Eletrodo de diamante dopado com boro 8000 ppm; 0,72 cm2 Eletrodos de carbono vítreo da Tokai Carbon Co Approximate potential ranges for platinum, mercury, carbon and boro-doped diamond electrodes 0 3.0 -3.0 1M H2SO4 Pt 1M NaOH 1M H2SO4 1M KCl Hg 1M NaOH 1M HClO4 C 0.1M KCl 0.5 M H2SO4 - 1.5 +2.5 BDD Glassy carbon electrode application Aplicação de EQM em sistema FIA Eletrodo base: Eletrodo de carbono vítreo Preparação do Eletrodo: Ciclagem de potencial entre -0,2 e 0,6 V (vs. Ag/Cl) em solução de 1,0 mmol L-1 FeCl3.6H2O e 10 mmol L-1 de K3[Fe(CN)6] Funcionamento [Fe(CN)6]4- [Fe(CN)6]3- + AA [Fe(CN)6]3- + e- [Fe(CN)6]4- Comportamento voltamétrico do sistema Aplicação de EQM em sistema FIA Anodic stripping voltammetric determination of copper(II) using a functionalized carbon nanotubes paste electrode modified with crosslinked chitosan Janegitz, B.C., Marcolino-Junior, L.H., Campana-Filho, S.P.,Faria, R.C., Fatibello-Filho,O. Sensors and Actuators B, 142, 260 (2009) Comparison: with and without carbon nanotube functionalization Anodic stripping voltammetry •80 % CNTs (w/w) + 20 % nujol (w/w) •-0.2V for 270 s • 25 mV Carbon Nanotubes Functionalized s-1 (B) 300 300 250 250 200 200 I/ A I/A (A) 150 100 150 100 50 50 0 0 -0.2 0.0 0.2 0.4 0.6 E/ V vs. Ag/AgCl 0.8 -50 -0.2 0.0 0.2 0.4 0.6 0.8 E / V vs. Ag/AgCl Figure XX - Linear voltammograms obtained with electrodes containing functionalized nanotubes not (A) and functionalized (B), in 0.1 mol L-1 NaNO3 solution in the presence of Cu2 + 9.0 x 10-5 mol L-1. 49 Anodic stripping voltammetry •-0.2V por 270 s •25 mV s-1 EPNM-QTS-ECH 30 D I/A 20 10 A 0 -10 -0.2 0.0 0.2 0.4 0.6 0.8 E/V vs. Ag/AgCl Figura XX -. Stripping voltammetry for EPN (A), EPNM-QTS (B), EPNM-QTS-GA (C) and EPNM-QTS-ECH (D) in 0.1 mol L-1 NaNO3 solution in the presence of Cu2 + 9.0 x 10-5 mol L-1., = 25mV s-1, a 25ºC. 50 Analytical Curve 300 6 12 250 8 200 1 4 I/ A I/ A 16 0 150 100 50 -4 0 -0.3 -0.2 -0.1 0.0 0.1 0.2 E/ V vs. Ag/AgCl Figura XX - Voltammograms obtained for the construction EPNM-QTS-ECH with 15% (w/w) QTS-ECH in 0.05 mol L-1 NaNO3 solution. -50 -0.2 0.0 0.2 0.4 0.6 0.8 E / V vs. Ag/AgCl Figura XX - Analytical curve: • 7.93 x 10-8 a 1.6 x 10-5 mol L-1 L D =1.06 x10-8 mol L-1 , LQ= 7.93 x 10-8 mol L-1 RSD= 3.12% Determination of Cu2+ Concentration de Cu (II) (mol L-1) Sample Urine samples Industrial Waste Method Method Erro relativo % comparative* proposed 0.50 ± 0.03 0.52 ± 0,09 4,.0 2.4 ± 0.2 2.3 ± 0.1 -4.1 3.5 ± 0.2 3.6 ± 0.1 1.0 10.7 ± 0.2 11.1 ± 0.1 3.6 Voltammetric determination of ciprofibrate using a glassy carbon electrode modified with functionalized carbon nanotube within a poly (allylamine hydrochloride) film o The ciprofibrate is a fibrate and present Antilipemic effect (lipid lowering); o Fibrates are indicated for patients who, after tests confirmed that the increase in endogenous triglecirideos is due to poor nutrition; o A possible interest in determining the ciprofibrate addition to quality control of drugs; CH3 O Cl Cl COOH CH3 Figure XXX - Ciprofibrate molecular estructure. Functionalization of MWCNTs in acid solution (H2SO4/HNO3 3:1) Carbon nanotubes dispertion in PAH solution [dispertion]=1mg mL-1 Film formation on the elcetrode by casting technique (20 μL) (a) (b) (c) (d) Figure XX - PAH SEM images (a) and (b); MWCNTs/PAH SEM image (c) and (d) Analytical curve 6 6 Linear equation: i= -0.700 + 4.75 x 104 x C i/A 4 2 4 0 0,84 0,91 0,98 1,05 1,12 Concentration range: 13.3 to132 mol L-1 i/A E/V vs. Ag/AgCl 2 Detection Limit: 8.34 mol L-1 0 0 40 80 [ciprofibrate]/mol L 120 -1 Figure XX - Analytical curve obteined for ciprofibrate determination in phosphate buffer solution 0,01 mol L-1 by VPD. = 12 mV s-1, A= 60 mV, t= 100 ms Table XX - Ciprofibrate determination in pharmaceuticals formulations using GCEMWCNTs/PAH and standard method Sample Label value (mg) DPV HPLC method method REc1 A 100 100 ± 3 98±5 2 B 100 99 ± 4 100±7 -1 C 100 99 ± 6 100±4 -1 D 100 100 ± 6 104±2 -4 REc1 = 100 x (VPD value – Reference method value) / Reference method value Frutas e vegetais empregados como fontes da PFO (POLIFENOL OXIDASE) e PER (PEROXIDADE) em bioreatores e biossensores. Abacate (Persea americana) Abobrinha (Cucurbita pepo) Alcachofra (Cynara scolymus L.) Berinjela Cara Batata inglesa (Solanum tuberosum) (Solanum melongena) (Dioscorea bulbifera) Jaca (Artocarpus integrifolia L.) Mandioca (Manihot utilissima) Nabo (Brassica campestre ssp.) Banana (Musa paradisiaca) Coco (Cocus nucifera L.) Pêssego (Prunus persica) Batata doce (Ipomoea batatas L. Lam.) Inhame (Alocasia macrorhiza) Rabanete (Raphanus sativus) Enzimas São proteínas que agem como catalisadores biológicos: enzima Composto A Composto B Centro ativo ou sítio catalítico Não há consumo ou modificação permanente da enzima Emil Fisher, década 50 Modelo chave-fechadura E e S se deformam quando em contato (alteração conformacional), para otimizar o encaixe Daniel Kosland, 1970 Modelo Encaixe induzido Biossensor para glicose - Radiometer® Fig. Esquema de um biossensor OH OH OH + + O2+ 3 H PFO + Fenol Catecol OH O OH + 1/2 O2 Catecol PFO O + o-quinona H2O H2O Screen-printed electrodes Low cost Portability Practicality 64 Screen-printed electrodes “screen-printed” or “silk-screen” Technology the possibility of mass production Extremely low cost Simplicity Complete electrochemical system Referen Count er ce Work Figura 5: Struture of screen printed electrodes 65 Screen-printed electrodes Substrates Work electrode Plastic materials (Polyester) Ceramics Metals Metalic films Nanoparticles •Addition Carbon nanotubes •Deposition Enzymes Polymers Complexation agents 66 New Materials Carbon nanotubes Copper Boron-doped diamond (BDD) Gold Carbon glassy (CG) Iridium Metallic films Antimony etc Bismuth Etc. 67 Bismuth film 2002 Vytras et al. Pauliukaite et al. 2003 Wang et al. Carbon paste modified with Bi2O3 Bismuth film electrode (BiFE) electrodeposited in CG 68 Bismuth film • Good cathodic potential window • Interference of dissolved oxygen is minimal • Low toxicity • Electrochemical behavior is similar to that of mercury 69 Bismuth film electrode MEV-FEG A) B) Figura 10: Micrographs of the BiFE A) 10000x B) 50000x 70 Bismuth film determination electrode for anodic stripping SWV lead B A C Bi deposit = Copper plate 3-electrodes scheme Insulating film Definition of the superficial area Ag deposit = Bi Film mini-sensor (A): PalmSens and (B): DropSens potentiostats and (C) BiSPE preparation Confecção do minissensor 120 °C durante 200 s FeCl3 0,50 mol L-1 em meio de HCl 0,10 mol L-1 durante 15-20 minutos. 72 Bismuth film electrode electrode tt-type connector for printers Bismuth redox process II 0,02 I / 0,01 I Bi3+ + 3eBi0 II Bi3+ Bi0 + 3e- 0,00 -0.30 V 0.08 V -0,01 -0,02 -0,03 -0,6 I -0,5 -0,4 -0,3 -0,2 -0,1 0,0 0,1 0,2 -1 E/ V vs. Ag/AgCl ( KCl 3,0 mol L ) Figura 7: Cyclic voltammogram for 0.02 mol L-1 Bi(NO3)3 in 0.10 mol L1 acetate buffer (pH 4,5) solution as electrolyte support; the work electrode is a platinum foil and scan rate of 10 mV s-1. Filme de bismuto -0.18 V vs. Ag/AgCl (3.0 mol L-1 KCl) during 200 s 0.02 mol L-1 Bi(NO3)3, 1.0 mol L-1 HCl in 0.15 mol L-1 Sodium citrate. 74 Determination of lead 16 28 12 20 pa / A pa / A 24 16 12 8 8 4 4 0 -0,8 -0,7 -0,6 -0,5 E / V vs. Ag/AgCl -0,4 0 0 1 2 2+ 3 4 5 -1 [Pb ] / mol L Anodic stripping voltammograms of 9.9 x 10-8 – 8.3 x 10-6 lead (LD of 5.8 x 10-8 M) in 0.1 M acetate buffer (pH 4.5), using square-wave mode. Deposition at 1.1 V for 2 min; pulse amplitude of 28 mV; increment of potential of 3 mV and frequency of 15 Hz. Bismuth film electrode (BiFE) for paraquat determination In 0.1 mol L-1 HAC pH 4,5, using differential pulse voltammetry. Figueiredo-Filho, L. C. et al, Electroanalysis, 22, 1260 (2010) DPV para determinação de Paraquat Besides of paraquat can be determined simultaneously Cd2+ e Pb2+. Determination of PQ in six natural water samples by BIFE and HMDE (reference). Samples* /µ mol L-1 *The HMDE BIFE ER (%) A1 59.03 ± 0.06 58.73 ± 0.03 -0.51 A2 58.74 ± 0.01 59.23 ± 0.00 0.84 A3 58.35 ± 0.03 57.41 ± 0.02 -1.61 A4 29.36 ± 0.08 29.56 ± 0.03 0.68 A5 29.23 ± 0.05 27.97 ± 0.02 -4.31 A6 27.95 ± 0.05 29.51 ± 0.02 5.58 SD (±) was calculated from three replicates. Confecção do minissensor Figura- Etapas da confecção do minissensor Filme de bismuto -0,18 V vs. Ag/AgCl (KCl 3,0 mol L-1) durante 200 s Bi(NO3)3 0,02 mol L-1, HCl 1,00 mol L-1 e citrato de sódio 0,15 mol L-1 Cola de prata Bright Silver Epoxy (BSE) + Gray Silver Hardener (GSH). Após a aplicação na superfície de cobre esperou-se 24 horas para a cura da cola 79 Atrazina • Atrazina (ATZ) (2-cloro-4-etilenodiamino-6isopropilamino-s-triazina) • Pertence a classe das triazinas • Composto polar, fracamente básico de coloração branca Cl N C2H5 HN N N NH CH CH2 3 Figura. Fórmula estrutural da Atrazina 80 Comportamento eletroquímico da Atrazina (ATZ) 0 I / -3 -6 -9 -1,2 -1,0 -0,8 -0,6 -0,4 -1 E / V vs. Ag/AgCl ( 3,0 mol L ) Figura 11- Voltamograma obtido para uma solução de Atrazina 4,00 x 10-5 mol L-1, utilizando tampão acetato 0,10 mol L-1, pH *4,5 em 15 % v/v de etanol como eletrólito suporte. * pH condicional Cl N CH3CH2NH Cl N N CH3 NHCH CH3 N H+ CH3CH2NH Cl N N+ H CH3 NHCH CH3 * e- CH3 CH3CH2NH N H NHCH CH3 N eCH3CH2NH N N CH3 + ClNHCH CH3 81
Download